Figure 8. ISP1 likely interacts with β‐tubulin in ookinete.
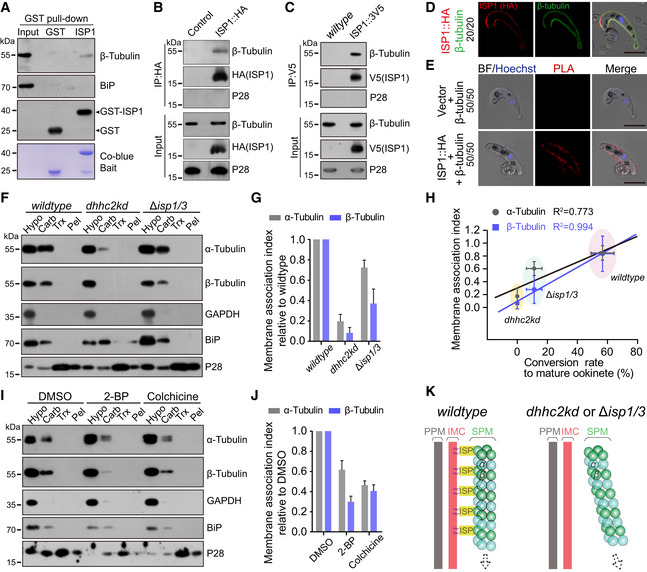
- GST‐ISP1 pulls down endogenous β‐tubulin from cell lysate of WT ookinetes. Lower panel indicates the Coomassie blue stain of recombinant GST and GST‐ISP1.
- Episomally expressed ISP1::HA immunoprecipitated with β‐tubulin in WT ookinetes. Episomal vector as control.
- Endogenous ISP1::3V5 immunoprecipitated with β‐tubulin in the TTS ookinetes.
- Co‐localization of ISP1::HA and β‐tubulin at ookinete periphery in IFA. Scale bar = 5 μm.
- Proximity ligation assay (PLA) detecting interaction of ISP1‐HA and β‐tubulin at ookinete. Episomal vector as control. Scale bar = 5 μm.
- Solubility assay detecting membrane association of α‐ and β‐tubulins in WT, dhhc2kd, and ∆isp1/3 parasites. GAPDH is a cytosolic soluble protein, and P28 is a plasma membrane integral protein.
- Quantification of α‐ and β‐tubulins membrane association in F. The membrane association index is defined as the ratio of Carb fraction to Hypo fraction in F and normalized to that of WT. Values are means ± SEM from three independent tests.
- Linear correlation of membrane association index of α‐tubulin and β‐tubulin with the conversion rate to mature ookinete among WT, dhhc2kd, and ∆isp1/3 parasites. Horizontal and vertical values are means ± SD.
- Membrane association of α‐ and β‐tubulins in WT ookinete culture after DMSO, 2‐BP, or colchicine treatment.
- Quantification of α‐ and β‐tubulins membrane association in I. Values are means ± SEM from three independent repeats.
- Model showing ISP1/ISP3 interaction with β‐tubulin for anchoring microtubules with IMC in the elongating ookinetes.
Source data are available online for this figure.
