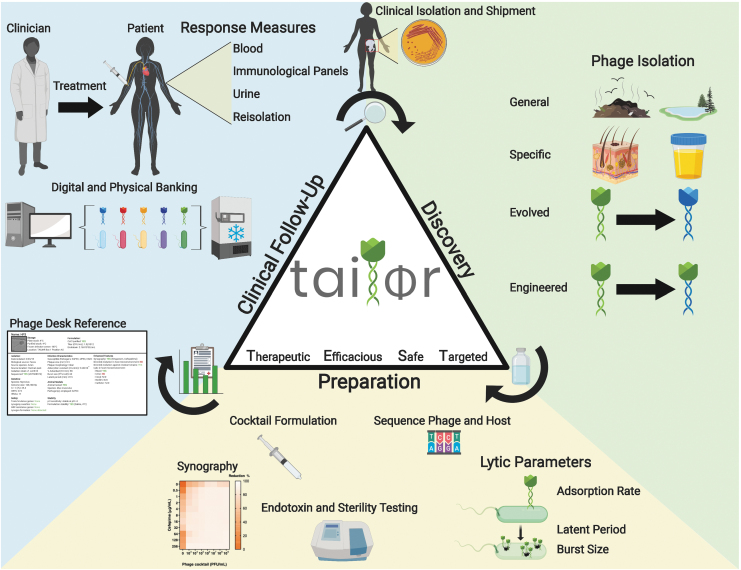
FIG. 2.
Overview of the discovery, preparation, and clinical follow-up process for TAILΦR. Phase 1 (phage discovery): Phages can be isolated from the environment, targeted samples, and/or selected by their enhanced activities; can be evolved by using a co-culture system to generate phages that are capable of infecting another host strain; and can be engineered genetically to have enhanced features. Phase 2 (phage preparation): To formulate a phage cocktail, phages are characterized based on their lytic parameters (adsorption rate, latent period, and burst size), sequenced, assayed for synergism with antibiotics (synography), and tested for endotoxin levels, sterility, and stability. A report about the qualified phage cocktail is then sent to the attending physician and is used for the analysis and approval by the Food and Drug Administration. If approved, the cocktail can be sent to the physician. Phase 3 (clinical follow-up): TAILΦR assists with examining therapeutic efficacy during and after treatment by determining phage titers from clinical samples, developing new phages if required, and re-testing bacterial isolates for phage sensitivity. Lastly, all discovered phages are catalogued (Phage Desk Reference) and stored for long-term usage. TAILΦR, Tailored Antibacterials and Innovative Laboratories for Phage (Φ) Research.