Table 3.
Preparation of Monocyclic Arene Oxides (l3)a
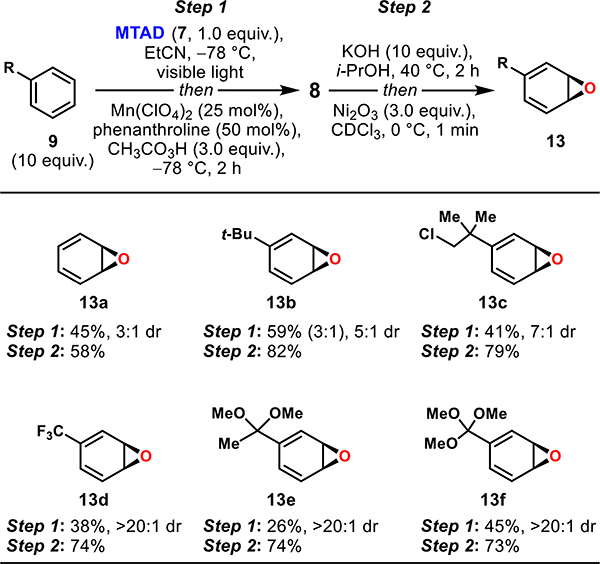 |
Standard reaction conditions. Step 1: MTAD (7, 1.0 mmol, 1.0 equiv), arene (9, 10 mmol, 10 equiv), EtCN (0.1 M), visible light, −78 °C, 12 h; then addition of Mn(ClO4)2/phenanthroline (25/50 mol %) in MeCN and CH3CO3H (3.0 mmol, 3.0 equiv), −78 °C, 2 h. Step 2: epoxide 8 (0.2 mmol, 1.0 equiv), KOH (2.0 mmol, 10 equiv), 40 °C, 2 h; then workup and exposure of crude to Ni2O3 (0.6 mmol, 3.0 equiv), CDO3 (0.1 M), 0 °C, 1 min. Reported yields of 8 (step 1) are of isolated products and the ratio of diastereoisomers and constitutional isomer (bracket) were determined by 1H NMR of the crude reaction mixtures. Reported yields of 13 (step 2) are based on 1H NMR integration relative to the internal standard (MeNO2).
