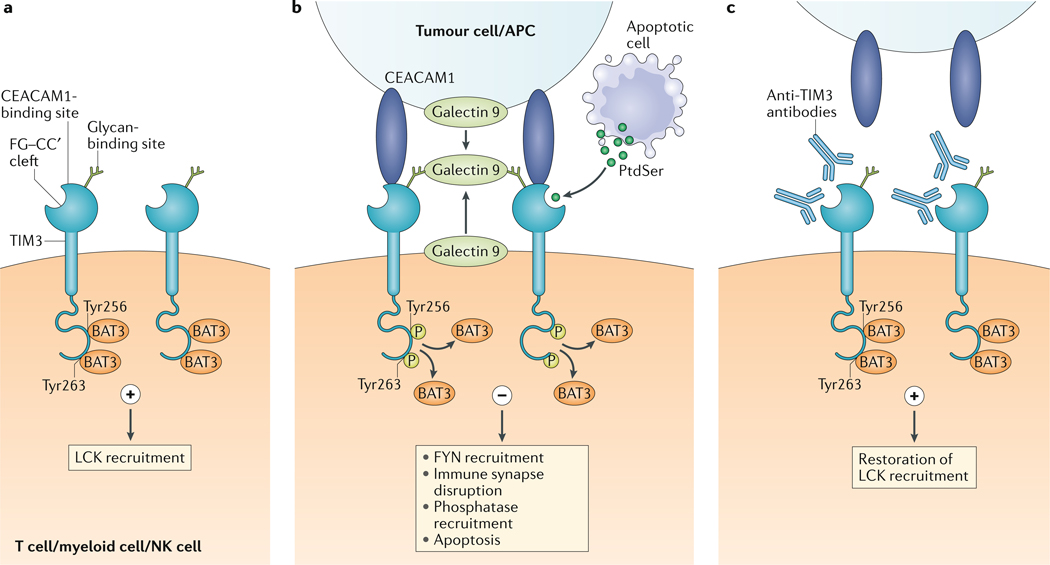
Fig. 1 |. models for TIm3–ligand interactions.
a | In its ligand-unbound form, T cell immunoglobulin and mucin domain-containing protein 3 (TIM3) interacts with HLA-B-associated transcript 3 (BAT3) and maintains T cell activation by LCK recruitment. b | Galectin 9 can be bound to the surface of tumour cells and can also be secreted by tumour cells, antigen-presenting cells (APCs) and other cells in the parenchyma, or can be secreted in autocrine fashion by TIM3-expressing cells. Its carbohydrate recognition domains bind to the glycan-binding site on TIM3. Galectin 9 has two carbohydrate recognition domains and can promote the oligomerization of TIM3, thus potentially facilitating the formation of other TIM3–ligand complexes such as carcinoembyronic antigen-related cell adhesion molecule 1 (CEACAM1)–TIM3. In addition, phosphatidylserine (PtdSer) released from apoptotic cells can bind the FG–CC′ cleft-binding site on TIM3. On binding to galectin 9 or CEACAM1, Tyr256 and Tyr263 in the intracellular domain of TIM3 are phosphorylated; this releases BAT3 and allows recruitment of the tyrosine kinase FYN. This results in the disruption of immune synapse formation and in phosphatase recruitment. Ultimately, the cell becomes anergic or undergoes apoptosis, which is mediated by intracellular calcium release. c | Most of the TIM3-targeted antibodies that facilitate antitumour immunity interfere with either CEACAM1 or PtdSer binding to TIM3, thus maintaining the TIM3–BAT3 interaction. NK cell, natural killer cell.