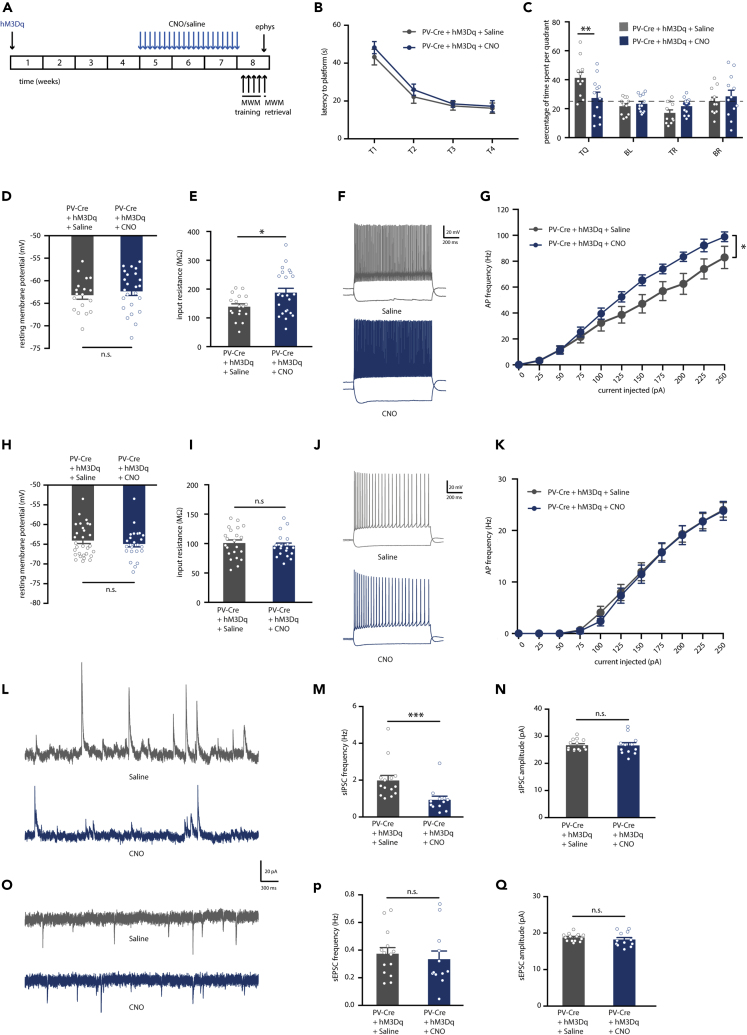
Figure 2.
Prolonged Activation of Hippocampal PV Interneurons Causes Spatial Memory Deficits, PV Neuron Hyperexcitability, and Network Imbalance on the Short Term
(A) PV-Cre mice were injected with hM3Dq virus at 8–10 weeks of age. After 4 weeks, CNO (1 mg/kg) or saline were i.p. injected daily for a period of 3 weeks. An MWM test was performed 48 h after discontinuation of CNO injections. Electrophysiological recordings were performed following the MWM test.
(B) Spatial learning was assessed measuring the latency to find the hidden platform on four consecutive training days (T1–4). Both PV-Cre mice that expressed hM3Dq and had received CNO injections or saline injections showed significant learning during training (training two-way repeated measures ANOVA: n = 11/12 mice per group, F3,60 = 40.50, p < 0.0001).
(C) During the 1-min probe trial, CNO-injected PV-Cre mice expressing hM3Dq spent significantly less time in the target quadrant (TQ) compared with PV-Cre mice receiving control saline injections (two-way ANOVA: n = 11/12 mice per group, F3,84 = 4.12, p = 0.009; post-hoc LSD test: ∗∗p = 0.002). Compared with chance level (dashed line), saline-injected PV-Cre mice spent significantly more time in the target quadrant, whereas CNO-injected PV-Cre mice did not (Student's t test: p < 0.01).
(D) PV interneuron resting membrane potential was not different between CNO-injected or saline-injected PV-Cre mice expressing hM3Dq (Student's t test: n = 18/24 cells from 5 mice per group, p = 0.514).
(E) PV interneuron input resistance was significantly increased in CNO-injected compared with saline-injected PV-Cre mice expressing hM3Dq (Student's t test: n = 18/24 cells from 5 mice per group, ∗p = 0.018).
(F) Voltage responses to 1 s hyperpolarizing or depolarizing current steps from a PV interneuron in saline-injected PV-Cre (gray) and CNO-injected PV-Cre (blue) mice.
(G) Average action potential (AP) frequency in response to 0–250 pA depolarizing current steps illustrating a significant increase in PV interneuron excitability in CNO-injected PV-Cre mice compared with saline-injected PV-Cre mice (group x current two-way repeated measures ANOVA: n = 18/24 cells from 5 mice per group, F1,30 = 7.13, p = 0.012).
(H and I) Pyramidal neuron resting membrane potential (H) and input resistance (I) were unaltered in CNO-injected PV-Cre mice compared with saline-injected PV-Cre controls (Student's t test: n = 29/25 cells from 5 mice per group, p = 0.437 and p = 0.534).
(J) Voltage responses to 1 s hyperpolarizing or depolarizing current steps from a pyramidal neuron in a saline-injected PV-Cre mouse (gray) or a CNO-injected PV-Cre mouse (blue).
(K) AP frequency in response to 0–250 pA depolarizing current steps illustrating no significant change in pyramidal neuron excitability in CNO-injected PV-Cre mice compared with saline-injected PV-Cre controls (group x current two-way repeated measures ANOVA: n = 29/25 cells from 5 mice per group, F1,38 = 0.02, p = 0. 882).
(L) Example traces of spontaneous inhibitory postsynaptic currents (sIPSC) recorded from hippocampal pyramidal neurons in saline-injected PV-Cre mouse (gray) or a CNO-injected PV-Cre mouse (blue).
(M) Decreased sIPSC frequency in CNO-injected PV-Cre mice compared with saline-injected controls (Mann-Whitney test: n = 14/12 cells from 4 mice per group, ∗∗∗p = 0.000).
(N) No alterations were observed in the amplitudes of sIPSCs (Mann-Whitney test: n = 14/12 cells from 4 mice per group, p = 0.526).
(O) Example traces of spontaneous excitatory postsynaptic currents (sEPSC) recorded from hippocampal pyramidal neurons in saline-injected PV-Cre mouse (gray) or a CNO-injected PV-Cre mouse (blue).
(P and Q) No significant alterations were observed in the frequency (P) or in the amplitude (Q) of sEPSCs in CNO-injected PV-Cre mice compared with saline-injected PV-Cre controls (Mann-Whitney test: n = 14/12 cells from 4 mice per group, p = 0.595 and p = 0.347). Values are mean ± SEM.