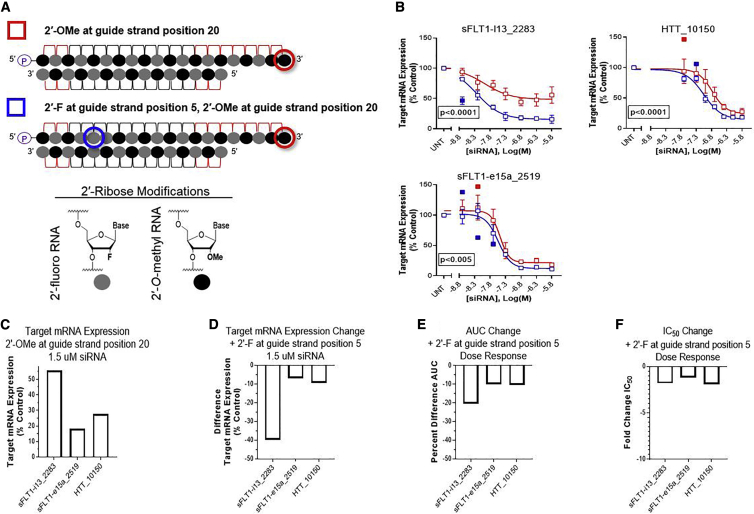
Figure 3.
2′-Fluoro at Position 5 of 20-mer Guide Strands Increases Activity of Fully Modified, Asymmetric siRNAs with 2′-O-Methyl at 3′ Guide Strand Termini
(A) Schematic representations of chemical modification scaffolds used; symbols next to each schematic are used in graphs shown in (B). Legend shows corresponding chemical structures for 2′-ribose modifications. (B) Dose-response results (n = 3, mean ± SD). Target name and start site of target sequence are indicated in each graph. HeLa cells were treated with siRNAs at concentrations shown for 72 h. mRNA levels were measured using the Dual-Glo Luciferase Assay System (sFLT1-i13, sFLT1-e15a) or Quantigene 2.0 RNA Assay (HTT) and calculated as a percent of those from untreated cell controls. Statistical outliers were excluded from analyses but are shown in the graphs as solid data points. p values displayed on each graph were calculated by two-way ANOVA. (C) Target mRNA expression with 1.5 μM of each siRNA with 2′-OMe at guide strand position 20. (D) Differences in target mRNA expression with 1.5 μM of each siRNA when 2′-F replaces 2′-OMe at guide strand position 5. (E) Percent differences in AUCs. (F) Fold changes in IC50 values, calculated by dividing the value obtained from siRNAs with 2′-F at guide strand position 5 by the value obtained from siRNAs with 2′-OMe at guide strand position 20; if this number was <1, then the negative reciprocal is shown. (D–F) Negative values indicate decreases in values when 2′-F replaces 2′-OMe at guide strand position 5.