Abstract
Rheumatoid arthritis (RA) is characterized by the production of anti-citrullinated protein antibodies (ACPAs). To gain insights into the relationship between ACPA-expressing B cells in peripheral blood (PB) and synovial tissue (ST), we sequenced the B cell repertoire in paired PB and ST samples from five individuals with established, ACPA+ RA. Bioinformatics analysis of paired heavy and light chain sequences revealed clonally-related family members shared between PB and ST. ST-derived antibody repertoires exhibited reduced diversity and increased normalized clonal family size compared to PB-derived repertoires. Functional characterization showed that seven recombinant antibodies (rAbs) expressed from subject-derived sequences from both compartments bound citrullinated antigens and immune complexes (ICs) formed using one ST-derived rAb stimulated macrophage TNF-α production. Our findings demonstrate B cell trafficking between PB and ST in subjects with RA and ST repertoires include B cells that encode ACPA capable of forming ICs that stimulate cellular responses implicated in RA pathogenesis.
Keywords: Rheumatoid Arthritis, ACPA, Antibody Repertoire Sequencing, Synovial Tissue, Peripheral Blood, Inflammation
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune synovitis that results in tender and swollen joints and bone erosions. Prior to the development of clinical RA, autoantibodies, such as rheumatoid factor and anti-citrullinated protein antibodies (ACPAs; detected by the clinical cyclic citrullinated peptide (CCP) test) are present in the serum of these patients [1–3]. However, the origin of these autoantibodies as well as their functional roles in promoting synovitis and joint-destruction are poorly understood. Here, we sought to enhance our understanding of the relationship between the antibodies present in peripheral blood (PB) and synovial tissue (ST) by sequencing the B cell repertoires of these two compartments in individuals with established RA.
ACPAs target citrullinated epitopes arising from post-translational modifications of arginine to citrulline by peptidyl arginine deiminase (PAD) [4]. Previous studies showed that citrullinated antigens and ACPAs play key roles in the pathogenesis of RA and that recombinant murine ACPAs can increase the severity of arthritis in mouse models [5, 6]. It is thought that ACPAs directly contribute to RA pathogenesis by stimulating macrophages. Inflamed ST in RA joints demonstrate an accumulation of macrophages and increased expression of pro-inflammatory cytokines, such as TNF-α [7], which promote synovitis [8]and osteoclastogenesis [9]. Consistent with these findings, anti-TNF-α therapeutics have proven successful for RA treatment [10]. Furthermore, immune complexes (ICs) composed of ACPAs derived from RA blood and citrullinated proteins can stimulate TNF-α production in macrophages derived from both blood [11]and RA synovial fluid [12]. These ACPA-containing ICs were shown to stimulate macrophages via FcγRII [12–15] and TLR4 [13, 14] indicating that the presence of ACPAs in ST and fluid may promote pathogenic TNF-α production from macrophages.
Functional germinal center (GC)-like structures surrounded by ACPA-producing plasma cells have been identified in inflamed ST from subjects with RA [16]. These findings suggest that pathogenic antibody-producing plasma cells may be generated locally. A previous study also showed that dominant B cell clones identified in the ST of subjects with early RA were enriched for the IGHV4–34 gene [17], which possesses intrinsic autoreactivity [18]. Further, in this previous study, select dominant clones were shared across knee joints of one patient, while little overlap was detected between ST and PB [17]. These previous studies established a complex relationship between PB- and ST-derived antibody repertoires; however, the extent of overlap and the functional roles of these antibody repertoires in RA pathology remains unclear. Therefore, in this study, we sought to further investigate the interplay between the B cells and ACPA present in PB and those present in ST, and to characterize the functional properties of individual PB and ST-derived ACPA to provide insight into their role in the pathogenesis of RA.
Here, we investigated the relationship between PB- and ST-derived antibody repertoires by sequencing individual B cells derived from matched PB and ST samples obtained from five individuals with established RA and positive responses in the CCP test (CCP+). To profile the antibody repertoire, we utilized a cell barcoding method that provides (i) full-length, paired heavy (HC) and light (LC) chain sequences, (ii) high-fidelity, error-free sequences, and (iii) precise quantification of the number of B cells belonging to a particular family/lineage [19]. Bioinformatics analysis revealed clonal expansions in both PB and ST, and that repertoires from the two compartments contained B cells derived from the same clonal family (i.e. shared clonal family members), although this overlap was limited. Functional characterization revealed that recombinant antibodies (rAbs) derived from clonal families as well as singletons from both compartments bind citrullinated antigens. Further, ICs composed of an ST-derived recombinant antibody and either citrullinated-H2A or citrullinated-H2B stimulated macrophages to produce TNF-α.
2. Materials and Methods
2.1. Human samples
All human samples were collected under Investigational Review Board (IRB)-approved protocols from Stanford University or University of Pittsburgh. Individuals undergoing total knee/hip arthroplasties were recruited for this study. RA subjects met the American College of Rheumatology 1987 and 2010 criteria [20, 21]. Three arthroplasty remnant tissue samples were obtained from CCP+ RA patients at Stanford University and subjects subsequently provided matched blood samples. Two additional remnant tissue were obtained from University of Pittsburgh with paired blood samples provided at the time of tissue donation. Monocyte-derived macrophages were generated from blood obtained from Stanford Blood Center.
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation with Ficoll-Paque™ PLUS (GE Healthcare Life Sciences) or Lympho-prep (Axis-shield). ST was isolated from the arthroplasty samples. Tissues were digested in a collagenase II preparation overnight or underwent a quick digestion in Liberase TL and DNAse using 30 minutes at 37°C with shaking before mashing through 70 μm sieve [22].
2.2. Sorting and sequencing individual B cells
Plasmablasts (CD19+CD3-IgD-CD14-CD20-CD27+CD38++) were single-cell sorted (BD FACSAria) from PBMCs as previously described [19, 23], while all CD19+ B cells were single-cell sorted from ST. PBMCs and ST-derived cells were stained with fluorophore-conjugated antibodies against CD19 (HIB19; Biolegend or SJ25C1; BD), CD3 (UCHT1; BD), IgD (IA6–2; BD), CD14 (MφP9; BD), CD20 (L27; BD), CD27 (CLB-27/1; LifeTechnologies or M-T271; BD), CD38 (HB7; BD), IgA (IS11–8E10; Miltenyi), and IgM (MHM-88; Biolegend). PBMCs from samples isolated at Stanford were co-stained with pooled citrullinated-peptide multimers comprised of 14 citrullinated peptides to identify ACPA-producing plasmablasts [24].
Sequencing of immunoglobulin gene-encoded mRNA from individual B cells was performed using cell barcodes as previously described [19, 23–25]. Unique well-specific oligonucleotide barcodes (TruGrade Oligos; IDT) were added during reverse transcription (Maxima Reverse Transcriptase; ThermoFisher Scientific). The cDNA from all 96 wells were pooled and HC and LC genes were amplified using gene-specific PCR primers, followed by paired-end sequencing (2×330) using Illumina MiSeq. Samples were prepared using two [25] or three [24]rounds of PCR amplification with gene-specific primers.
2.3. Bioinformatics analysis
Sequence data was processed, HC VDJ and LC VJ genes aligned using ImMunoGeneTics (IMGT) HighV-Quest [26], and phylogenetic trees generated as previously described [19, 23–25]. The number of IMGT-based somatic hypermutations within the V-region was used to compare mutation rates across subjects and tissues. A custom analysis pipeline assigned clonal families across all PB and ST sequences from a single patient based on identifying clones that used the same HC and LC VJ genes and showed 60% amino acid identity within HC and LC CDR3 regions (Levenshtein distance [27]). Clonal family sizes were normalized across compartments by the total number of sequences isolated from each compartment to determine the fraction of cells that were in each clonal family. Antibody repertoire diversity was evaluated using VDJtools [28] and represents the fraction of unique clonotypes isolated, where individual clonotypes are defined as sequences that utilize the same V and J gene and identical CDR3 nucleotide sequences. Diversity of each compartment was analyzed separately and therefore represents the number of unique clonotypes identified in each compartment divided by the total number of sequences isolated from that compartment. Lineage trees were generated by aligning immunoglobulin sequences to germline V genes by ClustalX2 [29], input to IgTree [30], and visualized in Graphviz 2.38 [31]. Network analysis of trees was performed using NetworkX [32].
2.4. Monoclonal antibody expression
A subset of subject-derived B cell antibody sequences representing clonal families identified in an individual compartment, shared between PB and ST, and/or derived from B cells binding to the citrullinated-peptide multimers were selected for recombinant expression. For consistency in antibody characterization assays, all antibodies were expressed on the human IgG1 Fc domain as previously described [24, 33].
2.5. Profiling antibody specificities
Recombinant monoclonal antibodies (rAbs; 15 μg/mL) were tested for CCP-binding activity using the QUANTA Lite® CCP3.1 IgG/IgA kit (Inova) according to the manufacturer’s protocol. After calculating activity units based on the protocol, the activity cutoff was set to three standard deviations above the average observed for the negative control antibodies across both ELISA plates. rAbs (50 μg/mL) were tested for binding specificity using a planar microarray as previously described [34, 35] to evaluate binding to >300 proteins/peptides. Fluorophore-conjugated anti-human IgG secondary antibodies were used to detect binding and median fluorescent intensities (MFIs) were determined from quadruplicate print spots. MFI values for controls and other replicated antibodies were averaged for display in the heatmap. Using the averaged values for the negative control antibodies, the maximum observed MFI value (MaxNC) and standard deviation (SD) across all antigens tested was determined to set the activity cutoff (~4900) at 10 SDs above this MaxNC.
Proteins were in vitro citrullinated by PAD derived from rabbit skeletal muscle (Sigma). To coat ELISA plates, proteins (1 μg/mL) – both native and in vitro citrullinated – were diluted in bicarbonate/carbonate buffer (pH 9.5). Recombinant antibody was added (12.5 μg/mL), and a biotinylated anti-human IgG secondary with High Sensitivity Streptavidin-HRP (ThermoFisher) was used in conjunction with 1-Step Ultra TMB-ELISA Substrate (ThermoFisher) for detection.
2.6. Macrophage stimulation assay
Macrophages were isolated and cultured from plated PBMCs by adhesion as previously described [13, 14, 24]. To form plate-bound ICs, plates were coated with 50 μL of protein (20 μg/mL), washed with PBS, blocked with 150 μL of 1% low-endotoxin BSA in PBS, and recombinant, subject-derived antibody (50 μg/mL) added to each well. After further washes, differentiated macrophages were added (50,000 cells/well) in media (5% FBS, without hMCSF). To block the FcγRII/CD32 and/or TLR4, cells were pre-incubated at 37°C for one hour with anti-CD32 (Stemcell Technologies, clone IV.3) and/or InSolution™ TLR4 Inhibitor, TAK −242 (EMD Millipore). Lipopolysaccharide (50 ng/mL; LPS, Sigma-Aldrich) directly added to the media of differentiated macrophages was used as a positive control. After 24-hour stimulation at 37°C, supernatants were harvested and TNF-α levels measured by ELISA (Peprotech).
2.7. Statistical analysis
Statistical analysis was performed in Prism 7 (GraphPad). One-way or two-way ANOVA followed by Tukey’s or Sidak’s test was used for multiple comparisons, and P < 0.05 was considered statistically significant.
3. Results
3.1. A subset of clonally expanded antibody families is shared between peripheral blood and synovial tissue
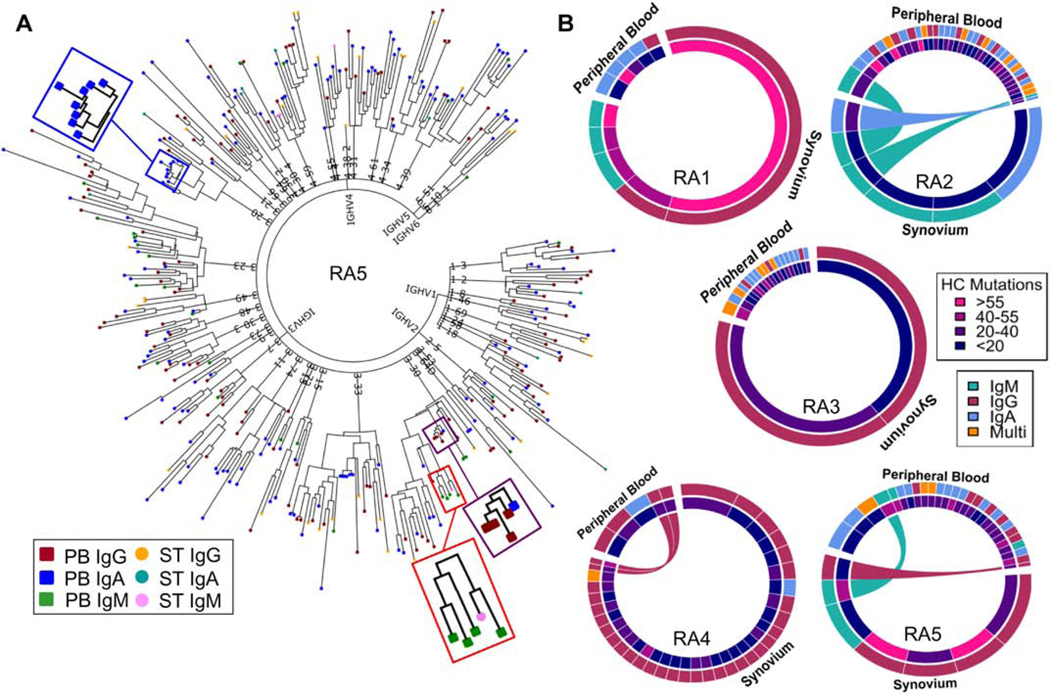
We sought to understand the relationship between B cells present in PB and ST in individuals with CCP+ RA and profile functional properties of PB- and ST-derived antibodies. Therefore, we utilized a cell-barcoding approach to sequence paired, full-length HC and LC variable region mRNA sequences from individual B cells isolated from PB and ST of five subjects with CCP+ RA. As previously described, this established approach combined with a custom bioinformatics pipeline identifies the error-corrected HC and LC sequences expressed by each cell, which are then aligned to immunoglobulin genes by IMGT, enabling expression of subject-derived recombinant antibodies for functional characterization [19, 23, 24, 36, 37]. We focused on capturing the active B cell response in PB, by sequencing the total plasmablast population and in a subset of patients co-stained with pooled citrullinated-peptide multimers to identify potential ACPA-producing plasmablasts. The resulting antibody repertoire datasets were used to construct phylogenetic trees to capture an overview of the repertoires in both PB and ST. Clonally expanded families, which were defined by a custom pipeline, and individual B cells without any clonally related family members (singletons) were present in the antibody repertoire sequence datasets (Fig. 1A). These phylogenetic trees demonstrate that certain families are comprised solely of a single immunoglobulin isotype (e.g. IgA), while others include members expressing multiple immunoglobulin isotypes (e.g. IgG/IgA). Further, the phylogenetic tree of the antibody repertoire for subject RA5 highlights that certain clonal expansions are shared across the two compartments, and one such clonal family identified was comprised of IgM-expressing B cells present in PB and ST (Fig. 1A; red box). The shared nature of these clonal families indicates that trafficking of B cells occurs between PB and ST in RA.
Fig. 1: Bioinformatics analysis identifies clonally expanded families, including those with representatives in peripheral blood and synovial tissue.
(A) A representative phylogenetic tree presents an overview of the antibody repertoire isolated from the peripheral blood (PB) and synovial tissue (ST) of subject RA5. Each leaf (colored by isotype and tissue) represents the concatenated HC and LC sequence isolated from a single B cell. While some sequences present as singletons, others cluster as clonally expanded families. Certain families are present only in PB and comprised of a single isotype (blue box); comprised of multiple isotypes (purple box); or present in multiple tissue compartments (red box). (B) Chord diagrams denote clonal expansions identified in PB and ST by bioinformatics analysis. The size of each section reflects the relative size of each family normalized by the number of sequences isolated from that compartment. Each section is colored by the number of mutations (inner ring) and isotype (outer ring). Expansions shared between ST and PB are connected by chords colored by isotype.
To further evaluate and summarize the identified clonal expansions across all the subjects, we constructed chord diagrams for each of the five patients (Fig. 1B). Across three of the four subjects with ≥40 antibody sequences derived from ST, we identified seven clonal families with representatives in both ST and PB compartments, indicating trafficking between tissues (Fig. 1B). Further, clonally expanded families in both compartments were comprised of different immunoglobulin isotypes and showed varied mutation rates (Fig. 1B). Normalizing the size of each family to the number of sequences in the respective compartments revealed that a larger percentage of total ST sequences than PB sequences represented clonal expansions. This suggests that the ST-derived repertoire is comprised of a more focused set of clonally-related B cells.
3.2. The peripheral blood antibody repertoire exhibits increased diversity
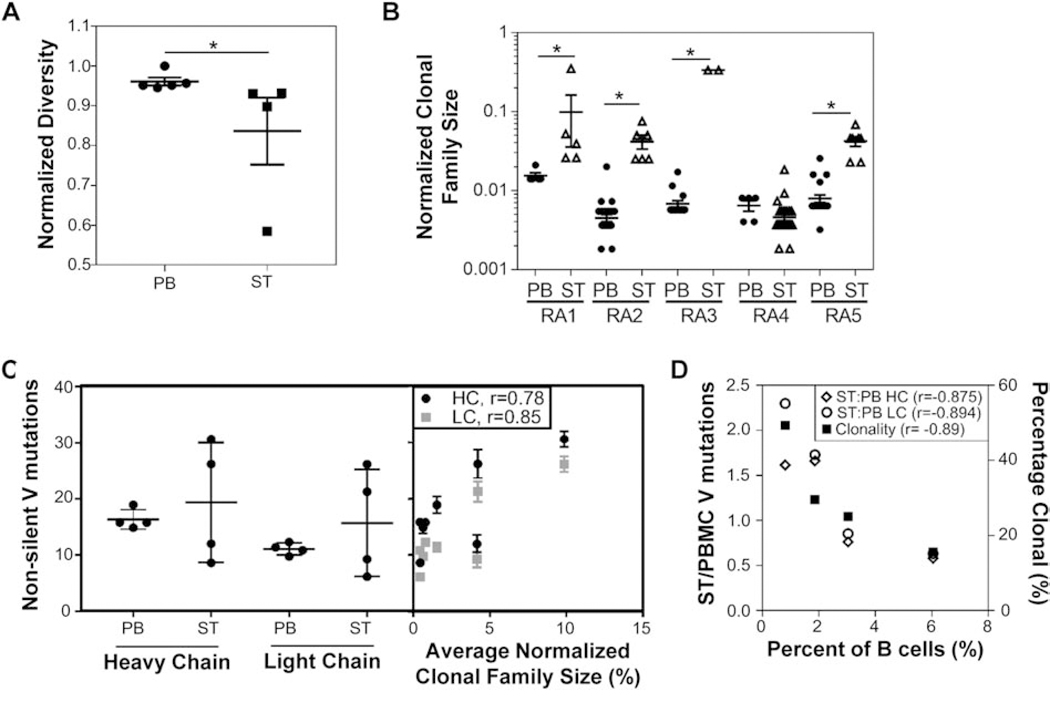
Extending upon our initial observation that the ST-derived repertoire appears more focused, we further investigated the similarities and differences between the PB and ST repertoires. We compared repertoire diversity across samples with ≥40 sequences (n = 5 for PB; and n = 4 for ST). Diversity (i.e. the fraction of unique clonotypes out of all sequences identified within each compartment) was significantly higher among the PB samples compared to ST (Fig. 2A). To quantitatively compare the degree of clonal expansions in the two compartments, we determined the normalized sizes of clonal families identified in each compartment and observed that individual clonal families comprised significantly larger fractions of sequenced ST repertoires than PB repertoires in 4 of the 5 subjects, further highlighting a more focused repertoire in ST (Fig. 2B). Mutational analysis revealed that some subjects exhibited a higher number of non-silent V gene mutations among the isolated ST sequences as compared to PB-derived sequences while others showed a reduced number of non-silent mutations in ST as compared to PB (Fig. 2C). However, further analysis demonstrated a significant positive correlation between the number of non-silent mutations and the average normalized clonal family size in both ST and PB repertoires (Fig. 2C). In contrast, we observed an inverse trend between the degree of infiltration, or percent of B cells out of the total ST-derived cells, and the ratio of the number of non-silent mutations in ST to PB repertoires as well as the percent clonality, which represents the percent of sequences belonging to a clonal family out of the total number of sequences (Fig. 2D). Together, these data indicate that increased B cell infiltration into ST leads to increased diversity (i.e. lower percent clonality), instead of larger clonal families. Since the sequences belonging to clonal families often possessed high levels of somatic hypermutation, the increased number of non-clonally expanded B cells with lower mutation levels led to a reduction in the average mutation levels that we observed in the ST repertoire compared to PB.
Fig. 2: The peripheral blood repertoire exhibits increased diversity with a reduced degree of clonal expansion.
(A) The diversity or fraction of unique clonotypes is higher among peripheral blood (PB)-derived (n = 5) sequences compared to synovial tissue (ST)-derived (n = 4) sequences. († - p < 0.05, Mann-Whitney U test). (B) ST-derived clonal families represent a larger portion of the total number of sequences isolated, as shown by the normalized clonal family size (i.e. number of members within a clonal expansion divided by the total number of sequences isolated). (* - p < 0.01, ANOVA followed by Sidak’s test compared to matched PB). (C) The average number of non-silent mutations is compared between PB and ST (left), highlighting a bifurcation in the subjects analyzed; however, this mutation rate significantly correlates with the average normalized clonal family size for each subject/tissue (right) (r – Pearson correlation coefficient). (D) The percent of B cells isolated from ST is compared with the ratio of V gene mutations in ST versus PB (left axis) and degree of clonality (right axis) for each sample. (r – Pearson correlation coefficient).
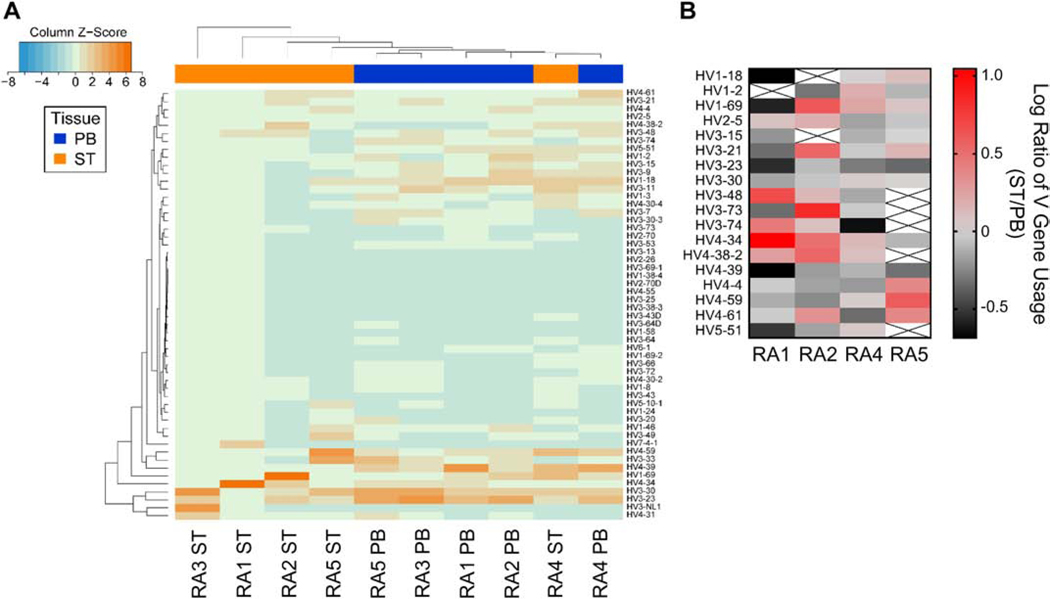
We next investigated V gene usages in the PB and ST repertoires from each subject to determine whether any selection bias existed within the ST repertoire. Hierarchical clustering based on V gene usage revealed closer relationships between PB repertoires from four subjects than to the repertoires from their respective ST samples. However, the matched PB and ST from RA4 clustered together (Fig. 3A). Additionally, certain V genes, such as 4–34 and 1–69, were more commonly utilized in ST as compared to PB in the four subjects with ≥40 ST sequences (Fig. 3B). These data indicate an altered preference for V genes between the two compartments. This enrichment for certain V genes in ST could indicate different mechanistic biases in the selection/expansion of antibodies in each compartment.
Fig. 3: Altered V gene usage is observed between synovial tissue and peripheral blood repertoires.
(A) The heatmap shows the overall frequency of V gene usage with unsupervised hierarchical clustering to evaluate degree of similarity among samples and tissues (peripheral blood (PB) = blue, synovial tissue (ST) = orange). (B) The ratio of V gene usage between ST and PB highlights certain V genes are more commonly observed among ST-derived sequences isolated from the four subjects with the deepest sequence coverage.
3.3. Clonal families comprised of peripheral blood-derived antibodies exhibit increased complexity
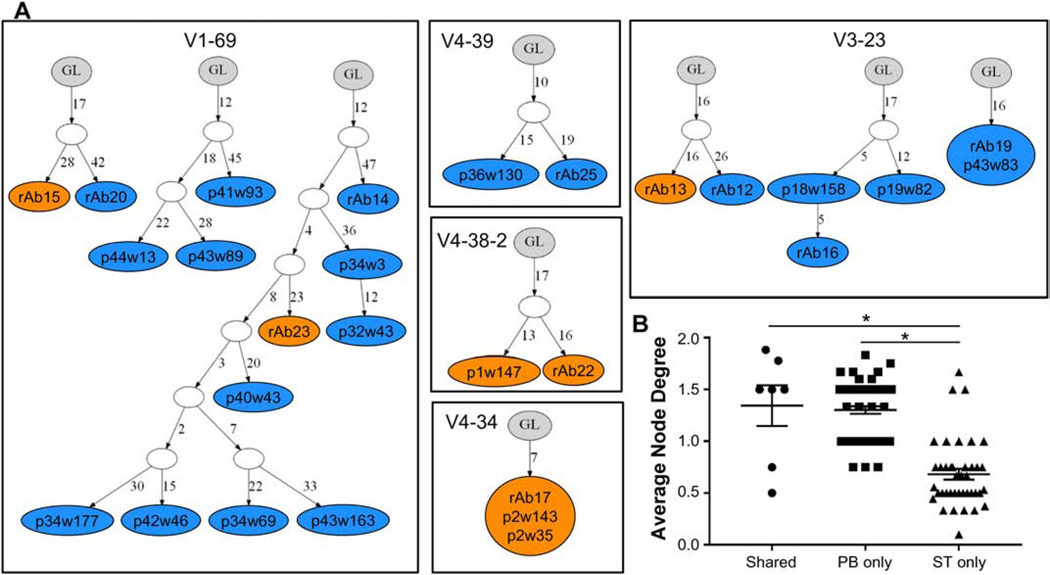
To gain further insights into the relationship between clonal expansions present in PB and ST, we evaluated the diversity and complexity of the clonal families with representatives in both compartments (shared) compared to clonal families with representatives found only in either PB or ST. IgTree was used to construct lineage trees for each clonal family identified in the three subjects for which shared families were observed. Each colored node in a tree represents a unique sequence within a clonal family. Individual antibodies are further identified within each node either by the plate and well ID of the cell from which the sequence was derived or by the number of the recombinantly expressed antibody (rAb). Representative lineage trees from RA2 demonstrate the variation in size among different clonal expansions with some including multiple members undergoing varied levels of affinity maturation, while others contain multiple cells expressing antibodies with identical sequences (multiple labels within a node; Fig. 4A). We compared the complexity of these clonal families by determining the average node degree for each clonal expansion, which represents the average number of connections from a specific node or antibody sequence. Families with representatives from PB, including those identified only in PB and those shared with ST, exhibited significantly higher complexity than the families with ST-only representatives (Fig. 4B). This finding reinforces our previous observation of increased diversity in the PB repertoire (Fig. 2A) and, in combination with the larger normalized clonal family sizes detected in ST (Fig. 2B), shows that the ST comprises a more focused repertoire and suggests that continued antigen stimulation in the periphery contributes to affinity maturation of the ACPA response.
Fig. 4: Clonal expansions containing members identified in peripheral blood exhibit increased complexity.
(A) Representative lineage trees from RA2 constructed using IgTree demonstrate clonal families shared across peripheral blood (PB) and synovial tissue (ST) and those only identified in PB or ST. Clonal families are grouped by HC V gene usage and labeled within each box. Each colored node represents a unique sequence identified from PB (blue) or ST (orange). Node labels indicate plate (p) and well (w) ID for a specific cell; rAb indicates an expressed antibody sequence. Nodes with multiple labels indicate multiple cells with identical sequences. (B) The complexity (average node degree) of each clonal family from the three subjects with expansions that had representatives in PB and ST is shown grouped by tissue type: representatives only in peripheral blood (PB only), only in synovial tissue (ST only) or in both compartments (Shared). (* - P < 0.01, ANOVA followed by Tukey’s test).
3.4. Peripheral blood- and synovial tissue-derived antibodies bind citrullinated antigens
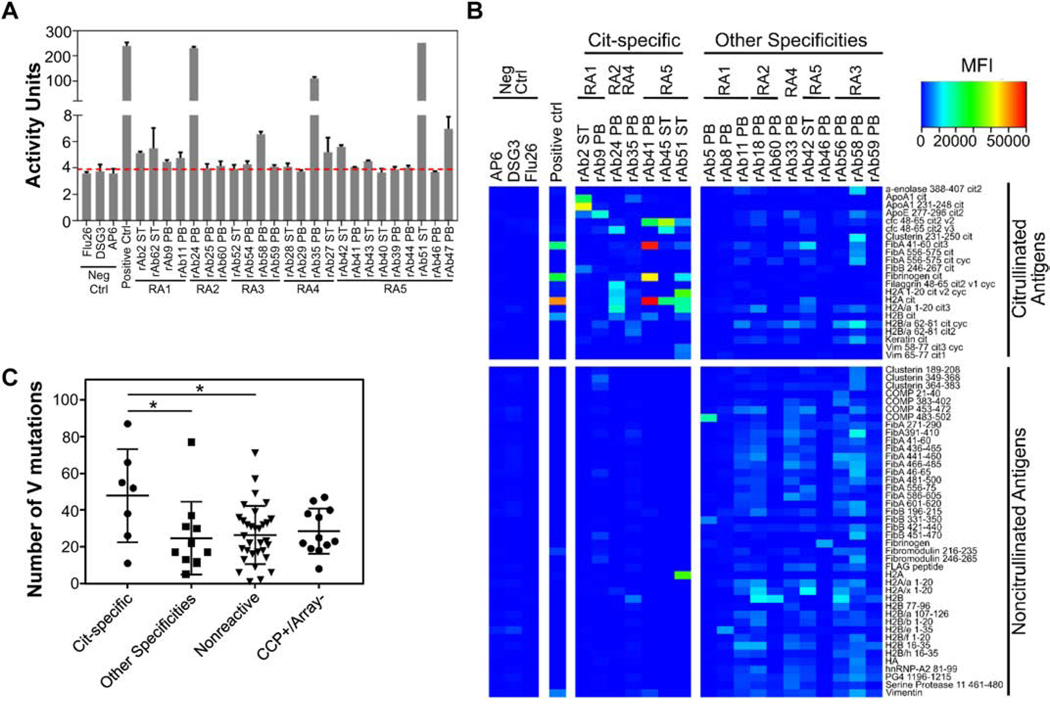
To analyze the functional properties of the antibodies identified in each compartment, we expressed a subset of paired HC and LC sequences derived from ST and PB repertoires as recombinant monoclonal antibodies (rAbs, Supplemental Table 1). The sequences selected for expression included representatives from clonal families found in either PB or ST and those shared between tissues. Additionally, sequences isolated from PB-derived plasmablasts that bound citrullinated peptide tetramers during sorting were also selected for recombinant expression. For technical reasons and to enable direct comparison of the binding and functional properties of the V regions identified, all recombinant antibodies were expressed on the human IgG1 Fc domain regardless of their original sequenced isotype. A subset of the PB- and ST-derived rAbs exhibited citrulline binding activity by the CCP3.1 ELISA (Fig. 5A). We further evaluated the binding specificity of the rAbs using an antigen microarray composed of >300 different peptides/proteins in both citrullinated and native forms. Seven distinct rAbs, including those derived from ST and PB, predominantly bound citrullinated antigens (cit-specific) including citrullinated forms of ApoA1, H2A and fibrinogen (Fig. 5B), demonstrating the ability of antibodies from both compartments to bind citrullinated antigens. An additional 11 rAbs either bound both citrullinated and native antigens or predominantly bound to non-citrullinated antigens (other specificities; Fig. 5B). The remaining rAbs did not appear to bind any of the peptides/proteins on the microarray (nonreactive). Therefore, these nonreactive antibodies captured from ST and the active plasmablast response from PB likely bind antigens not captured on our microarray. However, we focused our functional characterization on citrullinated antigens to better understand the role of ACPA in PB and ST in RA. One rAb bound citrullinated antigens by microarray but did not show CCP-binding activity by ELISA (Supplemental Table 1, Fig. 5A), indicating that the CCP3.1 ELISA may not be as sensitive for certain monoclonal antibodies. Conversely, a subset of the rAbs with some level of CCP reactivity by ELISA did not bind citrullinated antigens on the microarray (CCP+/Array-), suggesting these antibodies may bind citrullinated antigen(s) not captured by the microarray.
Fig. 5: Peripheral blood- and synovial tissue-derived recombinant antibodies bind citrullinated antigens.
Reactivity of recombinant antibodies (rAbs) derived from peripheral blood (PB) and synovial tissue (ST) is shown for (A) the CCP3.1 ELISA and (B) citrullinated and native peptides/proteins by planar antigen microarray. CCP3.1 ELISA activity cutoff (red dotted line) was determined based on negative control (Neg Ctrl) antibodies. (C) The number of HC V gene mutations varies across antibodies with different binding specificities: rAbs predominantly binding to citrullinated antigens by planar microarray (Cit-specific); rAbs predominantly binding non-citrullinated antigens or exhibiting binding to both citrullinated and non-citrullinated antigens by planar microarray (Other Specificities); rAbs with no significant binding to any tested antigens (Nonreactive); rAbs that exhibit reactivity by CCP3.1 ELISA but do not exhibit reactivity to citrullinated antigens by planar microarray (CCP+/Array-). (* - p < 0.05, ANOVA followed by Tukey’s test).
The cit-specific rAbs identified by microarray had, on average, a higher number of somatic hypermutations in the HC V region compared to both the nonreactive rAbs and the rAbs that bound both citrullinated and non-citrullinated antigens (Fig. 5C), which suggests that affinity maturation refines the specificity of antibodies to citrullinated antigens. However, there was no statistical difference between the number of HC V gene somatic hypermutations among the cit-specific rAbs identified by antigen microarray and the CCP+/Array-rAbs. This further suggests that the latter group of rAbs bind citrullinated antigens, but their targets were not represented on the antigen microarray.
3.5. Immune complexes containing a synovial tissue-derived antibody stimulate macrophage TNF-α production
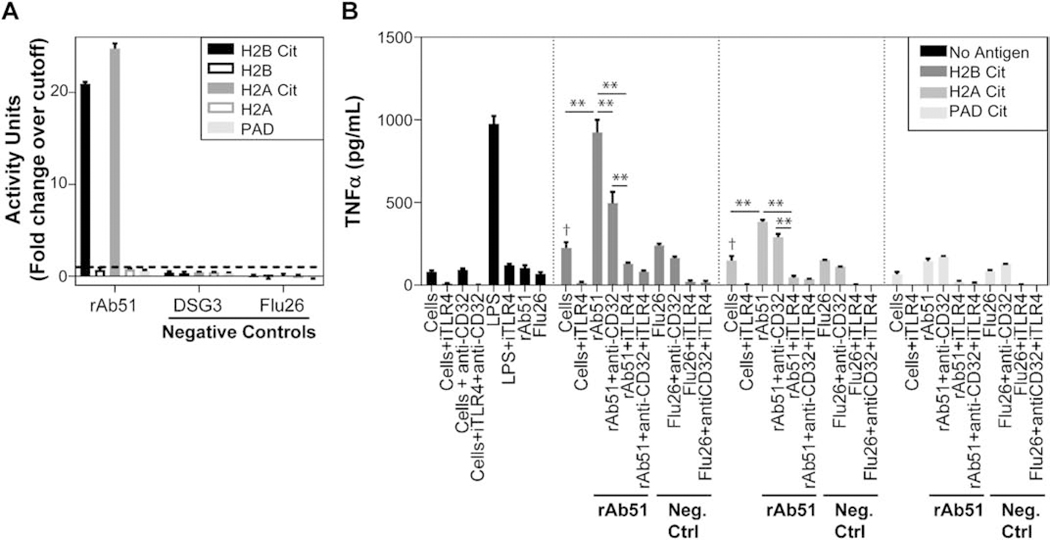
To enhance our understanding of the role of ACPA in RA pathology, we investigated the immunostimulatory properties of a subject-derived antibody that specifically bound citrullinated antigens. Previous studies implicated ACPA-containing immune complexes (ICs) in the stimulation of TNF-α production from macrophages [13, 14, 24]. Therefore, we tested whether ICs containing rAb51, an ST-derived, IgG antibody that predominantly bound citrullinated antigens (Fig. 5B), could stimulate a similar response in macrophages. We confirmed the specificity of rAb51 by ELISA, showing that it exhibited high levels of reactivity to the in vitro citrullinated forms of the full-length H2A and H2B proteins, but not the native forms (Fig. 6A). Stimulation of macrophages in culture with ICs comprised of rAb51 and either cit-H2B or cit-H2A resulted in significantly elevated levels of TNF-α production compared to stimulation with ICs containing a negative control antibody (Flu26) or stimulation with cit-H2A or cit-H2B alone (Fig. 6B). Furthermore, TNF-α levels following stimulation of macrophages with ICs containing cit-H2B and rAb51 were comparable to those following stimulation with LPS. This IC-mediated stimulation of TNF-α production was significantly reduced when macrophages were incubated with reagents that blocked CD32 (anti-CD32) and/or TLR4 (iTLR4) prior to their addition to plate-bound ICs (Fig. 6B). Interestingly, pre-incubation with iTLR4 showed a more significant blocking effect on TNF-α production than anti-CD32. We also confirmed that cit-H2A and cit-H2B, individually, increased production of TNF-α compared to cells alone, while rAb51 incubated with cells alone did not substantially increase TNF-α production (Fig. 6B).
Fig. 6: Immune complexes comprised of a synovial tissue -derived recombinant antibody stimulate TNF-α production from macrophages.
(A) The reactivity of the synovial tissue (ST)-derived rAb51 to in vitro citrullinated H2B and H2A is confirmed by ELISA. (B) TNF-α levels measured from cultured human macrophages are shown across a variety of conditions. Different levels of TNF-α were observed after incubation with immune complexes formed with either rAb51 or a negative control antibody (Flu26; Neg Ctrl) and citrullinated H2B, H2A, or PAD antigens. Some macrophages were pre-incubated with different inhibitors to block the Toll-like receptor 4 (iTLR4) and/or Fcγ receptor type II (anti-CD32) prior to addition to the plate. Lipopolysaccharide (LPS) was used as a positive control, and cells without the addition of antibodies were also used as controls. (** - p < 0.001, two-way ANOVA, † - p <0.05, one-way ANOVA compared to cells alone, followed by Tukey’s test).
We further evaluated whether residual PAD in the cit-H2A and cit-H2B preparations could be contributing to the macrophage stimulation by testing the ability for PAD, alone, to stimulate TNF-α production. However, we did not observe a significant increase in the levels of TNF-α in the presence of PAD or PAD and rAb51 together. Together, these findings demonstrate a mechanism by which this ST-derived, IgG antibody specifically interacts with citrullinated antigens to form ICs that stimulate macrophage TNF-α production through TLR4 and CD32 to varying degrees depending upon the citrullinated antigen in the IC. Thus, ACPAs present in ST can form ICs with citrullinated antigens to promote macrophage TNF-α production, which may contribute to RA pathology.
4. Discussion
The presence of ACPAs is a hallmark of RA and can be detected in serum years prior to development of clinical RA [2, 3]. However, the origin of ACPAs and their functional roles in promoting synovitis and joint-destruction are poorly understood. To gain insights into the relationship between ACPA-expressing B cells in the PB and ST, we profiled the antibody repertoires in these compartments by sequencing individual B cells from subjects with CCP+ RA. We identified clonal expansions in both PB and ST, a subset of which were shared between compartments. We demonstrated that the ST repertoires exhibited reduced diversity compared to the PB repertoires and showed that clonal families with representatives in PB were more complex than those in which we only identified representatives in the ST. Importantly, we found that the focused ST repertoire contains B cells that encode antibodies possessing the ability to bind to citrullinated antigens, form ICs, and stimulate macrophage TNF-α production. Together, these findings suggest that there is trafficking of B cell clonal families between PB and ST in RA, and that antibodies present in the ST possess properties which may be relevant for RA pathogenesis.
Our studies here demonstrate a complex relationship between the PB and ST B cell repertoires of subjects with established CCP+ RA. Clonal expansions were present in both compartments, and a small subset of clonal families were shared between PB and ST. This is consistent with a previous study demonstrating minimal overlap between the two compartments [17]. Further, the ST-derived B cell repertoire was more focused than the PB-derived plasmablast repertoire as indicated by: (i) the decreased antibody diversity of the ST repertoire, (ii) the greater percentage of B cell sequences identified in ST that comprised clonal families, and (iii) the decreased complexity of clonal families with representatives identified only in ST than those with representatives identified in PB. These findings are consistent with persistent peripheral antigen stimulation playing a role in affinity maturation of the ACPA response. Previous studies suggested that RA ACPA are generated in the lung mucosa [3, 38–40] and that ACPA cross react with microbes present in the mouth [41]. Additionally, we previously provided evidence of an ongoing IgA ACPA response leading to continued affinity maturation in individuals with established RA [24]. Since plasmablasts circulate transiently following an immune stimulus (e.g., damage-induced translocation of gingival microbiota), the presence of affinity-matured clonal families shared between the PB-derived plasmablast and ST-derived B cell populations implies that stimulation occurs continuously throughout chronic disease, rather than a single inciting event as occurs with acute viral infections for some autoimmune diseases. Thus, minimizing mucosal inflammation, either therapeutically or by lifestyle changes, may provide a benefit even in established RA.
Our analysis demonstrated altered V gene usage rates between the ST-derived sequences and those from PB. ST-derived sequences had higher usage rates of HC V genes 1–69 and 4–34, which are known to be associated with autoreactivity [42, 43]. It was previously reported that individuals with RA had an enrichment of HV4–34 usage in ST-derived antibodies [17], which has been shown to be intrinsically self-reactive [18, 44]. Usage of 1–69 is also enriched in clonal expansions in subjects with Sjögren’s syndrome and B-cell related cancers [44]. The enrichment of these HC V genes in ST-derived sequences suggests that there may be selection for antibodies with particular properties in ST in RA. Future studies focusing on mechanisms of selection of antibodies in ST may shed more light on the enrichment of specific antibody properties in ST, how focused antibody repertories arise in ST, and how the degree of B cell infiltration in ST may impact the ST-derived repertoire.
We demonstrated that rAbs derived from both RA PB plasmablasts and ST B cells specifically bound citrullinated antigens. Further, rAbs specific for citrullinated antigens had, on average, higher numbers of mutations than rAbs that bound both citrullinated and non-citrullinated antigens or those that were non-reactive. Consistent with our previous findings, these observations suggest that increased levels of somatic hypermutations drive epitope spreading of the ACPA B cell response [24]. Similarly, others recently demonstrated that ACPA from RA synovial fluids exhibited high levels of somatic hypermutations and broad cross-reactivity to modified peptides indicative of a sequential selection process [45].
We demonstrated that one of the ST-derived antibodies, rAb51, formed ICs with citrullinated antigens H2A and H2B, and these ICs stimulated macrophage TNF-α production. Production of pro-inflammatory cytokines by synovial macrophages contributes to inflammation and joint destruction and remodeling in RA [7]. Thus, these findings indicate that ACPA produced by ST B cells have the capacity to promote pathogenic responses in RA. As rAb51 contained a high number of somatic hypermutations (>60 mutations in the HC V region), this further supports our previous findings that continued affinity maturation further enhances the immunostimulatory properties of the ACPA B cell response [24]. Another study recently demonstrated that a synovial fluid derived antibody with high mutation levels promoted osteoclastogenesis and bone erosion in vitro [45]. However, this previous study did not identify the specific properties of this antibody that mediated this effect. In the present study, we probed the specific functional properties of an ST-derived ACPA and demonstrated that it can form ICs that stimulate TNF-α production through TLR4 and CD-32. Overall, our findings indicate an underlying mechanism for the role of ACPA in promoting inflammation in CCP+ RA joints through the activation of synovial macrophages.
This study has multiple potential limitations. First, limited sequencing depth may have hampered our ability to identify small clonal families and to comprehensively characterize the repertoires in ST and PB. Therefore, we may have missed other shared clonal families or additional members that would have increased the complexity of families in ST. Second, the small number of patients included in this study prevents us from understanding the full extent to which PB and ST repertoires differ. A larger study with different subtypes of RA subjects (e.g. CCP+ versus CCP-) may provide additional insights into the antibody repertoires present in ST and PB and the role of ACPA. Additionally, some ST and PB samples were not time matched, which may have reduced our ability to detect B cells expressing shared antibodies between the compartments. Third, our selection of CD19+ B cells in the ST as compared to analysis of plasmablasts from PB adds potential biases to directly comparing their antibody repertoires. Fourth, for technical reasons and to be consistent in our evaluation of the antigen-binding specificity for subject-derived antibodies and reduce artefact that may be introduced due to differential properties of the Fc regions of different antibody isotypes, all rAbs were expressed on an IgG1 backbone. Nevertheless, it is likely that isotype-specific properties contribute to the pathogenicity of antibodies in RA. Finally, we evaluated antibody specificity against a set of >300 proteins/peptides; however, the identification of a subset of rAbs exhibiting CCP+ reactivity by ELISA that were nonreactive to citrullinated peptides via microarray suggests that certain specificities may have been missed. Therefore, further expansion of this set of proteins/peptides may help determine additional specificities for ACPA in both PB and ST.
5. Conclusions
In summary, sequencing B cell antibody repertoires from both PB and ST from individuals with CCP+ RA revealed that clonal families are shared between the two compartments. The increased diversity of the PB antibody repertoire and complexity of clonal families comprised of PB-derived B cells suggest that persistent antigen stimulation in the periphery helps drive the ACPA B cell response. Importantly, we demonstrated that antibodies derived from the ST can bind citrullinated antigens, and furthermore, stimulate the production of TNF-α from macrophages. Taken together, our findings suggest that B cells can traffic between PB and ST in subjects with established CCP+ RA, and that ST-derived B cells can produce antibodies with immunostimulatory properties that may contribute to the pathogenic inflammatory responses that mediate synovitis in RA.
Supplementary Material
Acknowledgements
We thank the Stanford Functional Genomics Facility and Stanford Shared FACS Facility for their assistance.
Funding: Funding was provided by NIH NIAMS R01 AR063676, NIH NIAID U19 AI11049103, and NIH NIAID U01 AI101981 to WHR. SEE received support from NIH NIAID 5T32 AI07290-30.
Footnotes
Conflict of interest: William Robinson is a Founder, member of the Board of Directors, and consultant to Atreca, Inc. Serra Elliott is an employee of Biodesy, Inc. Sarah Kongpachith is an employee of and owns stock in AbbVie Inc. Lisa Blum is an employee of Bolt Biotherapeutics, Inc. All other authors declare no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD, Merkel PA, Pillemer SR, Reveille JD, Stone JH, National Arthritis Data W, Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I, Arthritis Rheum, 58 (2008) 15–25. [DOI] [PubMed] [Google Scholar]
- [2].Holers VM, Demoruelle MK, Kuhn KA, Buckner JH, Robinson WH, Okamoto Y, Norris JM, Deane KD, Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction, Nat Rev Rheumatol, 14 (2018) 542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Demoruelle MK, Bowers E, Lahey LJ, Sokolove J, Purmalek M, Seto NL, Weisman MH, Norris JM, Kaplan MJ, Holers VM, Robinson WH, Deane KD, Antibody Responses to Citrullinated and Noncitrullinated Antigens in the Sputum of Subjects With Rheumatoid Arthritis and Subjects at Risk for Development of Rheumatoid Arthritis, Arthritis Rheumatol, 70 (2018) 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang S, Wang Y, Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis, Biochim Biophys Acta, 1829 (2013) 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, Holers VM, Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis, J Clin Invest, 116 (2006) 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Uysal H, Bockermann R, Nandakumar KS, Sehnert B, Bajtner E, Engstrom A, Serre G, Burkhardt H, Thunnissen MM, Holmdahl R, Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis, J Exp Med, 206 (2009) 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR, Macrophages in rheumatoid arthritis, Arthritis Res, 2 (2000) 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Drexler SK, Kong PL, Wales J, Foxwell BM, Cell signalling in macrophages, the principal innate immune effector cells of rheumatoid arthritis, Arthritis Res Ther, 10 (2008) 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schett G, Teitelbaum SL, Osteoclasts and Arthritis, Journal of Bone and Mineral Research, 24 (2009) 1142–1146. [DOI] [PubMed] [Google Scholar]
- [10].Ma X, Xu S, TNF inhibitor therapy for rheumatoid arthritis, Biomed Rep, 1 (2013) 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Clavel C, Ceccato L, Anquetil F, Serre G, Sebbag M, Among human macrophages polarised to different phenotypes, the M-CSF-oriented cells present the highest pro-inflammatory response to the rheumatoid arthritis-specific immune complexes containing ACPA, Ann Rheum Dis, 75 (2016) 2184–2191. [DOI] [PubMed] [Google Scholar]
- [12].Laurent L, Clavel C, Lemaire O, Anquetil F, Cornillet M, Zabraniecki L, Nogueira L, Fournie B, Serre G, Sebbag M, Fcgamma receptor profile of monocytes and macrophages from rheumatoid arthritis patients and their response to immune complexes formed with autoantibodies to citrullinated proteins, Ann Rheum Dis, 70 (2011) 1052–1059. [DOI] [PubMed] [Google Scholar]
- [13].Sokolove J, Zhao X, Chandra PE, Robinson WH, Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor, Arthritis Rheum, 63 (2011) 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sohn DH, Rhodes C, Onuma K, Zhao X, Sharpe O, Gazitt T, Shiao R, Fert-Bober J, Cheng D, Lahey LJ, Wong HH, Van Eyk J, Robinson WH, Sokolove J, Local Joint inflammation and histone citrullination in a murine model of the transition from preclinical autoimmunity to inflammatory arthritis, Arthritis Rheumatol, 67 (2015) 2877–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, Sebbag M, Serre G, Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen, Arthritis Rheum, 58 (2008) 678–688. [DOI] [PubMed] [Google Scholar]
- [16].Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, Spencer J, Pitzalis C, Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium, PLoS Med, 6 (2009) e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Doorenspleet ME, Klarenbeek PL, de Hair MJ, van Schaik BD, Esveldt RE, van Kampen AH, Gerlag DM, Musters A, Baas F, Tak PP, de Vries N, Rheumatoid arthritis synovial tissue harbours dominant B-cell and plasma-cell clones associated with autoreactivity, Ann Rheum Dis, 73 (2014) 756–762. [DOI] [PubMed] [Google Scholar]
- [18].Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, Sanz I, Regulation of inherently autoreactive VH4–34 B cells in the maintenance of human B cell tolerance, J Clin Invest, 108 (2001) 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tan YC, Kongpachith S, Blum LK, Ju CH, Lahey LJ, Lu DR, Cai X, Wagner CA, Lindstrom TM, Sokolove J, Robinson WH, Barcode-enabled sequencing of plasmablast antibody repertoires in rheumatoid arthritis, Arthritis Rheumatol, 66 (2014) 2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. , The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis, Arthritis Rheum, 31 (1988) 315–324. [DOI] [PubMed] [Google Scholar]
- [21].Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G, 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative, Arthritis Rheum, 62 (2010) 2569–2581. [DOI] [PubMed] [Google Scholar]
- [22].Donlin LT, Rao DA, Wei K, Slowikowski K, McGeachy MJ, Turner JD, Meednu N, Mizoguchi F, Gutierrez-Arcelus M, Lieb DJ, Keegan J, Muskat K, Hillman J, Rozo C, Ricker E, Eisenhaure TM, Li S, Browne EP, Chicoine A, Sutherby D, Noma A, Accelerating Medicines Partnership RASLEN, Nusbaum C, Kelly S, Pernis AB, Ivashkiv LB, Goodman SM, Robinson WH, Utz PJ, Lederer JA, Gravallese EM, Boyce BF, Hacohen N, Pitzalis C, Gregersen PK, Firestein GS, Raychaudhuri S, Moreland LW, Holers VM, Bykerk VP, Filer A, Boyle DL, Brenner MB, Anolik JH, Methods for high-dimensional analysis of cells dissociated from cryopreserved synovial tissue, Arthritis Res Ther, 20 (2018) 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lu DR, Tan YC, Kongpachith S, Cai X, Stein EA, Lindstrom TM, Sokolove J, Robinson WH, Identifying functional anti-Staphylococcus aureus antibodies by sequencing antibody repertoires of patient plasmablasts, Clin Immunol, 152 (2014) 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Elliott SE, Kongpachith S, Lingampalli N, Adamska JZ, Cannon BJ, Mao R, Blum LK, Robinson WH, Affinity Maturation Drives Epitope Spreading and Generation of Proinflammatory Anti-Citrullinated Protein Antibodies in Rheumatoid Arthritis, Arthritis Rheumatol, 70 (2018) 1946–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kinslow JD, Blum LK, Deane KD, Demoruelle MK, Okamoto Y, Parish MC, Kongpachith S, Lahey LJ, Norris JM, Robinson WH, Holers VM, Elevated IgA Plasmablast Levels in Subjects at Risk of Developing Rheumatoid Arthritis, Arthritis & Rheumatology, 68 (2016) 2372–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alamyar E, Giudicelli V, Li S, Duroux P, Lefranc M-P, IMGT/HighV-QUEST: the IMGT® web portal for immunoglobulin (IG) or antibody and T cell receptor (TR) analysis from NGS high throughput and deep sequencing, Immunome research, 8 (2012) 26. [Google Scholar]
- [27].Levenshtein VI, Binary Codes Capable of Correcting Deletions, Insertions and Reversals, Soviet Physics Doklady, 10 (1966) 707–710. [Google Scholar]
- [28].Shugay M, Bagaev DV, Turchaninova MA, Bolotin DA, Britanova OV, Putintseva EV, Pogorelyy MV, Nazarov VI, Zvyagin IV, Kirgizova VI, Kirgizov KI, Skorobogatova EV, Chudakov DM, VDJtools: Unifying Post-analysis of T Cell Receptor Repertoires, PLoS Comput Biol, 11 (2015) e1004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG, Clustal W and Clustal X version 2.0, Bioinformatics, 23 (2007) 2947–2948. [DOI] [PubMed] [Google Scholar]
- [30].Barak M, Zuckerman NS, Edelman H, Unger R, Mehr R, IgTree: creating Immunoglobulin variable region gene lineage trees, J Immunol Methods, 338 (2008) 67–74. [DOI] [PubMed] [Google Scholar]
- [31].Gansner ER, North SC, An open graph visualization system and its applications to software engineering, Software-Practice & Experience, 30 (2000) 1203–1233. [Google Scholar]
- [32].Hagberg AA, Schult DA, Swart PJ, Exploring Network Structure, Dynamics, and Function using NetworkX, Proceedings of the 7th Python in Science Conference (SciPy2008), (2008) 11–15. [Google Scholar]
- [33].Nair N, Feng N, Blum LK, Sanyal M, Ding S, Jiang B, Sen A, Morton JM, He XS, Robinson WH, Greenberg HB, VP4- and VP7-specific antibodies mediate heterotypic immunity to rotavirus in humans, Science translational medicine, 9 (2017) eaam5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hueber W, Kidd BA, Tomooka BH, Lee BJ, Bruce B, Fries JF, Sonderstrup G, Monach P, Drijfhout JW, van Venrooij WJ, Utz PJ, Genovese MC, Robinson WH, Antigen microarray profiling of autoantibodies in rheumatoid arthritis, Arthritis Rheum, 52 (2005) 2645–2655. [DOI] [PubMed] [Google Scholar]
- [35].Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, Edison JD, Gilliland WR, Tibshirani RJ, Norris JM, Holers VM, Robinson WH, Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis, PLoS One, 7 (2012) e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Blum LK, Adamska JZ, Martin DS, Rebman AW, Elliott SE, Cao RRL, Embers ME, Aucott JN, Soloski MJ, Robinson WH, Robust B Cell Responses Predict Rapid Resolution of Lyme Disease, Front. Immunol., 9 (2018) 1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kongpachith S, Lingampalli N, Ju CH, Blum LK, Lu DR, Elliott SE, Mao R, Robinson WH, Affinity Maturation of the Anti-Citrullinated Protein Antibody Paratope Drives Epitope Spreading and Polyreactivity in Rheumatoid Arthritis, Arthritis Rheumatol, 71 (2019) 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, Harrington AR, Kolfenbach JR, Striebich CC, Pham QN, Strickland CD, Petersen BD, Parish MC, Derber LA, Norris JM, Holers VM, Deane KD, Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity?, Arthritis Rheum, 64 (2012) 1756–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, Kulik L, Robinson WH, Arend WP, Thompson PR, Holers VM, N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis, J Immunol, 186 (2011) 4396–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, Weisman MH, Solomon JJ, Fischer A, Okamoto Y, Kelmenson LB, Parish MC, Feser M, Fleischer C, Anderson C, Mahler M, Norris JM, Kaplan MJ, Cherrington BD, Holers VM, Deane KD, Anti-Citrullinated Protein Antibodies Are Associated With Neutrophil Extracellular Traps in the Sputum in Relatives of Rheumatoid Arthritis Patients, Arthritis Rheumatol, 69 (2017) 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li S, Yu Y, Yue Y, Liao H, Xie W, Thai J, Mikuls TR, Thiele GM, Duryee MJ, Sayles H, Payne JB, Klassen LW, O’Dell JR, Zhang Z, Su K, Autoantibodies From Single Circulating Plasmablasts React With Citrullinated Antigens and Porphyromonas gingivalis in Rheumatoid Arthritis, Arthritis Rheumatol, 68 (2016) 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vencovsky J, Zd’arsky E, Moyes SP, Hajeer A, Ruzickova S, Cimburek Z, Ollier WE, Maini RN, Mageed RA, Polymorphism in the immunoglobulin VH gene V1–69 affects susceptibility to rheumatoid arthritis in subjects lacking the HLA-DRB1 shared epitope, Rheumatology (Oxford), 41 (2002) 401–410. [DOI] [PubMed] [Google Scholar]
- [43].Mockridge CI, Rahman A, Buchan S, Hamblin T, Isenberg DA, Stevenson FK, Potter KN, Common patterns of B cell perturbation and expanded V4–34 immunoglobulin gene usage in autoimmunity and infection, Autoimmunity, 37 (2004) 9–15. [DOI] [PubMed] [Google Scholar]
- [44].Henry Dunand CJ, Wilson PC, Restricted, canonical, stereotyped and convergent immunoglobulin responses, Philos Trans R Soc Lond B Biol Sci, 370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Steen J, Forsstrom B, Sahlstrom P, Odowd V, Israelsson L, Krishnamurthy A, Badreh S, Mathsson Alm L, Compson J, Ramskold D, Ndlovu W, Rapecki S, Hansson M, Titcombe PJ, Bang H, Mueller DL, Catrina AI, Gronwall C, Skriner K, Nilsson P, Lightwood D, Klareskog L, Malmstrom V, Recognition of Amino Acid Motifs, Rather Than Specific Proteins, by Human Plasma Cell-Derived Monoclonal Antibodies to Posttranslationally Modified Proteins in Rheumatoid Arthritis, Arthritis Rheumatol, 71 (2019) 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.