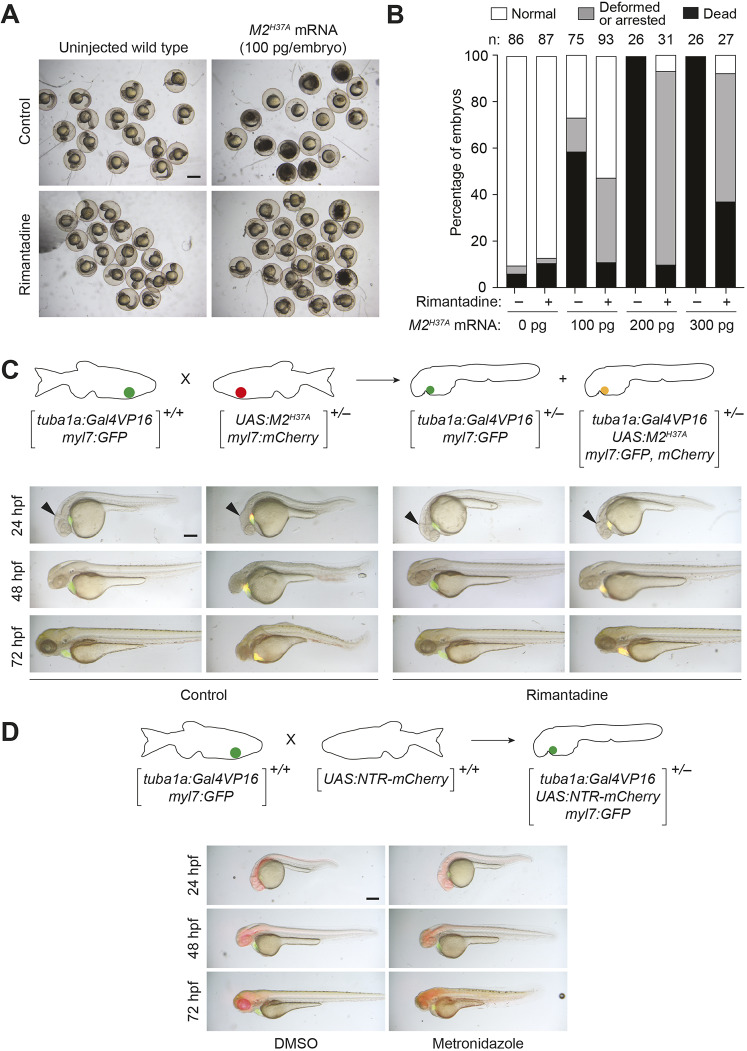
Fig. 1.
M2H37A channel variant is toxic to zebrafish embryos and ablates neurons more rapidly than NTR. (A) Wild-type zygotes were injected with the designated amount of M2H37A mRNA and cultured in the absence or presence of 100 μg/ml rimantadine. Representative bright-field micrographs of 24 hpf embryos from two independent experiments are shown. Scale bar: 500 μm. (B) Phenotypic distributions of embryos injected with the designated amount of M2H37A mRNA. The embryos were scored at 24 hpf, and the number of embryos (n) per condition from two or three independent experiments is indicated. Statistical analyses: χ2=389, d.f.=14, P<0.00001. (C) Comparison of Tg(tuba1a:Gal4VP16;myl7:GFP) and Tg(tuba1a:Gal4VP16; UAS:M2H37A;myl7:gfp,mCherry) embryos cultured in the absence or presence of 100 µg/ml rimantadine. Neuronal expression of M2H37A induces loss of the midbrain-hindbrain boundary (arrowheads) at 24 hpf and increasingly severe CNS deficits as development continues. Treatment with rimantadine starting at 10 hpf rescues these neuronal defects in Tg(tuba1a:Gal4VP16;UAS:M2H37A;myl7:gfp,mCherry) embryos. Representative bright-field and epifluorescence micrographs (overlays) from three independent experiments are shown. (D) Comparison of Tg(tuba1a:Gal4VP16;UAS:NTR-mCherry;myl7:GFP) embryos cultured with 5 mM metronidazole or an equivalent amount of DMSO, starting at 10 hpf. Metronidazole-treated embryos first exhibit CNS defects at 48 hpf. Representative bright-field and epifluorescence micrographs (overlays) from two independent experiments are shown. All embryos are shown in lateral view, anterior left. Scale bars: 250 µm. Statistics for the observed phenotypes are in Table S1.