Abstract
Zinc, an essential micronutrient, affects the heart by modulating cardiomyocyte oxidative stress and maintaining myocardial structure, among other mechanisms. In cross-sectional studies, patients with heart failure have often had zinc deficiencies, suggesting effects on the ongoing pathogenesis of heart failure. Low plasma and myocardial zinc levels may cause reversible cardiomyopathy in patients who have nutritional deficiencies.
We present the case of a 24-year-old woman with anorexia nervosa and new-onset heart failure whose depressed left ventricular systolic function improved after zinc supplementation. To our knowledge, this is the first report of low plasma zinc levels as the chief cause of cardiomyopathy that resolved after zinc supplementation.
Keywords: Cardiomyopathies/blood; disease progression; female; heart failure/blood/diet therapy/etiology/physiopathology; micronutrients/therapeutic use; nutritional physiological phenomena; treatment outcome; ventricular dysfunction, left; zinc/blood/deficiency/therapeutic use
The micronutrient zinc is a cofactor for antioxidant reactions in myocardial tissue and helps to maintain myocardial structure. In cross-sectional studies, zinc deficiency has frequently been found in patients with heart failure (HF), and investigators have suggested its influence in ongoing pathogenesis consequent to oxidative stress.1–5 Furthermore, zinc supplementation has reversed myocardial oxidative stress and apoptosis in patients who were nutritionally deficient after gastric bypass.6 We present the case of a young woman whose new-onset HF was most likely caused by zinc deficiency.
Case Report
In February 2013, a 24-year-old black woman with anorexia nervosa, chronic anemia (baseline hemoglobin, 9.5–11 g/dL), and major depression was admitted to the cardiac care unit with congestive symptoms and low cardiac output that necessitated inotropic support. She had no notable family medical history. Three months earlier, she had been admitted for failure to thrive after several months of poor oral intake that had caused a 30-lb weight loss and metabolic abnormalities. Initial laboratory results included normal levels of thiamine, folate, and selenium; however, her zinc levels were low at 23 μg/dL (normal range, 70–120 μg/dL). She had a prolonged hospital stay while she underwent tube-feeding and received antidepressant therapy. A transthoracic echocardiogram (TTE), obtained to investigate subjective dyspnea, revealed a left ventricular ejection fraction (LVEF) of 0.60 and nothing unusual. She was discharged from the hospital with plans for outpatient mental health follow-up.
Two weeks before the current admission, the patient reported progressive dyspnea and cough, which were attributed to respiratory tract infection and were treated unsuccessfully with azithromycin. On the day of presentation, her mother noted that she had a disturbed gait, right-limb weakness, facial drooping, and slurred speech. The diagnosis was acute right-sided middle cerebral artery stroke. The patient was given intravenous tissue plasminogen activator, and she gradually recovered neurologic function. Hypercoagulability study results revealed nothing of note, and no dysrhythmias were detected. However, physical examination and auscultation revealed prominent jugular venous pulsations, a soft right ventricular heave, and a left ventricular (LV) S3. A TTE showed a dilated LV with globally reduced function, an LVEF of 0.10, an LV end-diastolic dimension of 4.6 cm, a left atrial dimension of 4 cm, and an estimated right ventricular systolic pressure of 60 mmHg.
Results of right-sided heart catheterization included a right atrial pressure of 18 mmHg (normal, 2–6 mmHg), a pulmonary artery systolic pressure of 29 mmHg (normal, 15–30 mmHg), a pulmonary capillary wedge pressure of 16 mmHg (normal, 8–12 mmHg), a Fick cardiac output of 2.59 L/min (normal, 4–8 L/min), and a cardiac index of 1.71 L/min/m2 (normal, 2.5–4 L/min/m2). Cardiac magnetic resonance revealed normal chamber thicknesses and dimensions, severe biventricular failure, and a pericardial effusion of moderate size. No fresh or organized thrombi were seen in the cardiac chambers. Endomyocardial biopsy samples showed no acute inflammatory or infiltrative changes, viral inclusions, or adenovirus or herpes simplex virus after immunohistochemical staining. A reverse transcriptase polymerase chain reaction assay was negative for influenza A and B. Of note, no staining was obtained for changes associated with cardiomyopathy secondary to zinc deficiency, such as increased levels of superoxide dismutase or the protein LC3-II (a marker of autophagy).6
The patient was started on conventional therapy for advanced HF. The cause of new-onset cardiomyopathy in this young woman was not obvious, so the treatment team searched the medical literature and consequently tested her zinc levels. These were consistently low at 34 μg/dL. She was started on an oral zinc supplement (220 mg/d) and was encouraged to increase her dietary intake. Four weeks later, she was discharged from the hospital on a therapeutic regimen of carvedilol (12.5 mg 2×/d), lisinopril (10 mg/d), spironolactone (50 mg/d), torsemide (10 mg/d), hydralazine (10 mg/d), isosorbide dinitrate (10 mg 3×/d), and zinc (220 mg/d).
The patient's LVEF improved to 0.25 after one month and to 0.35 after 3 months. Zinc supplementation was discontinued at 4 months by a practitioner who was unaware of earlier therapeutic decisions, and the remaining therapy continued. After 6 months on this regimen, the patient's zinc levels recovered only partially to 58 μg/dL, and her LVEF remained at 0.35. While not taking zinc, she had another period of poor food intake; her zinc level decreased to 44 μg/dL, and supplementation was resumed. One year later, during which time she had continued zinc therapy, the patient's HF symptoms had resolved, her LVEF had improved to 0.60, and her zinc level had increased to 62 μg/dL.
Discussion
Zinc deficiency appears to have caused our patient's cardiomyopathy; prescribing a zinc supplement and medically managing her heart failure (and anorexia nervosa) resulted in her recovery. Other factors were unlikely to have caused cardiomyopathy. Imaging and endomyocardial biopsy results were inconsistent with infiltrative cardiomyopathy; serologic and histopathologic evaluations suggested no viral myocarditis; and this young patient's clinical course included no early reversal of biventricular dysfunction or findings on imaging of apical ballooning with basal sparing, making takotsubo cardiomyopathy unlikely. Her improved zinc levels and subsequently improved LVEF after therapy are evidence that zinc deficiency was the probable cause of cardiomyopathy. The HF therapy may also have contributed to myocardial recovery; however, her LV systolic function was poor at 4 months, coinciding with discontinued zinc supplementation during antiremodeling therapy.
Myocardial tissue needs zinc in small quantities to maintain the extracellular structure formed by matrix metalloproteinases7,8 and to serve as a cofactor in free radical oxidation reactions catalyzed by superoxide dismutase. When chronic inflammation is present, plasma zinc deficiency may lead to a relative deficiency in antioxidant enzymes, ultimately contributing to apoptosis and myocardial necrosis (Fig. 1).9,10
Fig. 1.
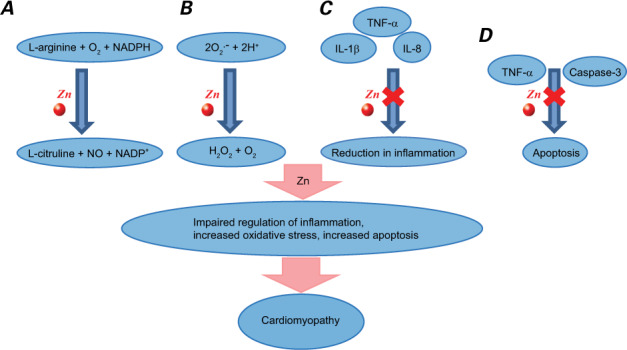
Diagram shows the function of zinc (Zn) in the heart and the proposed impact of zinc deficiency on cardiomyopathy. A) Zinc is a cofactor for nitric oxide (NO) synthase, a key component of endothelial health and vasodilation. B) Zinc is a crucial part of superoxide dismutase, which reduces superoxide anions and oxidative stress in cells. C) Zinc positively mediates gene expression of interleukin-2 and interferon-γ and negatively mediates tumor necrosis factor-α (TNF-α), interleukin-1β (IL-β), and interleukin-8 (IL-8). Deficiency leads to impaired immunity and increased inflammation. D) Zinc reduces the activity of TNF-α and caspase-3, preventing apoptotic signals.
H+ = hydrogen proton; H2O2 = hydrogen peroxide; NADPH = nicotinamide adenine dinucleotide phosphate; O2 = oxygen
To our knowledge, this is the first report of low plasma zinc levels as the chief cause of cardiomyopathy that resolved after zinc supplementation. When Frustaci and colleagues6 compared plasma and myocardial zinc levels in a group of patients who had malabsorption-related dilated cardiomyopathy with those levels in a group of patients who had idiopathic dilated cardiomyopathy and in a control group of patients who had nondilated cardiomyopathy, the group with malabsorption-related cardiomyopathy had lower zinc levels than did the other groups. These investigators also evaluated the effect of 6 months of zinc/selenium infusion therapy on zinc levels and LV function in 18 patients with malabsorption-related cardiomyopathy. These values improved significantly in the 10 patients who received the infusions, but not in the 8 patients who did not.6
Because severe zinc deficiency may induce reversible cardiomyopathy, zinc levels should be evaluated when patients with severe nutritional deficiencies present with HF. The general clinical usefulness of zinc supplementation in patients with HF warrants additional investigation.
Contributor Information
Behnood Bikdeli, Cardiovascular Research Foundation, New York, New York 10019.
Aakriti Gupta, Cardiovascular Research Foundation, New York, New York 10019.
Daniel Louis Jacoby, Section of Cardiovascular Medicine, Department of Internal Medicine, Yale New Haven Hospital, New Haven, Connecticut 06510.
References
- 1.Shokrzadeh M, Ghaemian A, Salehifar E, Aliakbari S, Saravi SS, Ebrahimi P. Serum zinc and copper levels in ischemic cardiomyopathy. Biol Trace Elem Res. 2009;127(2):116–23. doi: 10.1007/s12011-008-8237-1. [DOI] [PubMed] [Google Scholar]
- 2.Ripa S, Ripa R, Giustiniani S. Are failured cardiomyopathies a zinc-deficit related disease? A study on Zn and Cu in patients with chronic failured dilated and hypertrophic cardiomyopathies. Minerva Med. 1998;89(11–12):397–403. [PubMed] [Google Scholar]
- 3.Topuzoglu G, Erbay AR, Karul AB, Yensel N. Concentrations of copper, zinc, and magnesium in sera from patients with idiopathic dilated cardiomyopathy. Biol Trace Elem Res. 2003;95(1):11–7. doi: 10.1385/bter:95:1:11. [DOI] [PubMed] [Google Scholar]
- 4.Oster O. Trace element concentrations (Cu, Zn, Fe) in sera from patients with dilated cardiomyopathy. Clin Chim Acta. 1993;214(2):209–18. doi: 10.1016/0009-8981(93)90112-h. [DOI] [PubMed] [Google Scholar]
- 5.Ghaemian A, Salehifar E, Jalalian R, Ghasemi F, Azizi S, Masoumi S et al. Zinc and copper levels in severe heart failure and the effects of atrial fibrillation on the zinc and copper status. Biol Trace Elem Res. 2011;143(3):1239–46. doi: 10.1007/s12011-011-8956-6. [DOI] [PubMed] [Google Scholar]
- 6.Frustaci A, Sabbioni E, Fortaner S, Farina M, del Torchio R, Tafani M et al. Selenium- and zinc-deficient cardiomyopathy in human intestinal malabsorption: preliminary results of selenium/zinc infusion. Eur J Heart Fail. 2012;14(2):202–10. doi: 10.1093/eurjhf/hfr167. [DOI] [PubMed] [Google Scholar]
- 7.Weber KT, Weglicki WB, Simpson RU. Macro-and micronutrient dyshomeostasis in the adverse structural remodelling of myocardium. Cardiovasc Res. 2009;81(3):500–8. doi: 10.1093/cvr/cvn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efeovbokhan N, Bhattacharya SK, Ahokas RA, Sun Y, Guntaka RV, Gerling IC, Weber KT. Zinc and the prooxidant heart failure phenotype. J Cardiovasc Pharmacol. 2014;64(4):393–400. doi: 10.1097/FJC.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal PK, Kirshenbaum LA. A relative deficit in antioxidant reserve may contribute in cardiac failure. Can J Cardiol. 1990;6(2):47–9. [PubMed] [Google Scholar]
- 10.McKeag NA, McKinley MC, Woodside JV, Harbinson MT, McKeown PP. The role of micronutrients in heart failure. J Acad Nutr Diet. 2012;112(6):870–86. doi: 10.1016/j.jand.2012.01.016. [DOI] [PubMed] [Google Scholar]


