Abstract
PURPOSE
Performance status (PS) is a key factor in oncologic decision making, but conventional scales used to measure PS vary among observers. Consumer-grade biometric sensors have previously been identified as objective alternatives to the assessment of PS. Here, we investigate how one such biometric sensor can be used during a clinic visit to identify patients who are at risk for complications, particularly unexpected hospitalizations that may delay treatment or result in low physical activity. We aim to provide a novel and objective means of predicting tolerability to chemotherapy.
METHODS
Thirty-eight patients across three centers in the United States who were diagnosed with a solid tumor with plans for treatment with two cycles of highly emetogenic chemotherapy were included in this single-arm, observational prospective study. A noninvasive motion-capture system quantified patient movement from chair to table and during the get-up-and-walk test. Activity levels were recorded using a wearable sensor over a 2-month period. Changes in kinematics from two motion-capture data points pre- and post-treatment were tested for correlation with unexpected hospitalizations and physical activity levels as measured by a wearable activity sensor.
RESULTS
Among 38 patients (mean age, 48.3 years; 53% female), kinematic features from chair to table were the best predictors for unexpected health care encounters (area under the curve, 0.775 ± 0.029) and physical activity (area under the curve, 0.830 ± 0.080). Chair-to-table acceleration of the nonpivoting knee (t = 3.39; P = .002) was most correlated with unexpected health care encounters. Get-up-and-walk kinematics were most correlated with physical activity, particularly the right knee acceleration (t = −2.95; P = .006) and left arm angular velocity (t = −2.4; P = .025).
CONCLUSION
Chair-to-table kinematics are good predictors of unexpected hospitalizations, whereas the get-up-and-walk kinematics are good predictors of low physical activity.
INTRODUCTION
Accurate assessment of an oncology patient’s performance status (PS) is paramount for informing therapeutic decision making, whether it be to predict response and tolerability to treatment or determine eligibility for clinical trials. However, the 2 conventional scales used to measure PS—the Karnofsky Performance Status (KPS) scale and the Eastern Cooperative Oncology Group (ECOG) ranking—are observational and thus inherently limited in their precision. KPS and ECOG scales show discordance between health care professional observers, as well as between health care professionals and patients.1,2 In cases with patient-physician disagreement, physicians are likely to rate patients as better performing than patients are likely to rate themselves.2-4 Moreover, patients who have discordant ratings with their physicians have a 16% increase in risk of death, underscoring the importance of identifying a high-resolution means of assigning PS.4
CONTEXT
Key Objective:
Can oncologists use a motion sensor system in a clinic visit to predict treatment tolerability in patients with solid tumors receiving highly emetogenic chemotherapy?
Knowledge Generated:
Chair-to-table kinematics, as measured by Microsoft Kinect, are good predictors of unexpected hospitalizations, whereas get-up-and-walk kinematics, as measured by Microsoft Kinect, are good predictors of low physical activity.
Relevance:
Our findings suggest a motion-capture system as one tool with which oncologists can make more precise assessments of treatment tolerability in the clinical setting. This will better inform oncologists about clinical decision making, improve patient treatment options and outcomes, and expand eligibility criteria in oncologic clinical trials.
In addition, a patient’s PS plays a key role in his or her treatment trajectory. Reduced PS is associated with poor prognosis, diminished quality of life, and increased risk of sepsis and chemotherapy-induced nausea and vomiting, two conditions that can cause unexpected hospitalizations.5-7 Randomized clinical trials (RCTs) for anticancer treatment commonly use PS as an inclusion criterion, with few stratifying by PS. Although systematic reviews do not show PS to confer direct benefit in treatment efficacy, there is evidence that patients with reduced PS suffer greater toxicities that effectively diminish the clinical utility of the drug.8,9 Pooled analyses of patients with metastatic colorectal cancer show that reduced PS is associated with poorer survival and that patients with an ECOG PS of > 2 are more likely to experience nausea, vomiting, and 60-day all-cause mortality.10,11 Patients with lung cancer with reduced PS receiving cisplatin-based treatment overwhelmingly suffer disease-related grade III to IV hematologic toxicities.12 This pattern is paralleled in newer immunotherapy trials: More than 50% of patients with melanoma were not eligible for major RCTs on the basis of ECOG PS > 2, but those with reduced PS who do receive immunotherapy outside of prospective trials have reduced median survival and are more likely to be hospitalized within the last month of life.13,14
In geriatric oncology, frailty is also a consideration in predicting response to chemotherapy. Defined as diminished physical reserve with increased vulnerability, frailty has been demonstrated to be an independent predictor of survival in colorectal cancer.15 Similar to the measurement of ECOG and KPS, assessment of frailty is imperfect. Multiple tools have been proposed to characterize frail geriatric oncology patients; however, there has been no standardization and current tools may be time consuming.16 An objective, in-clinic tool to predict chemotherapy toxicity and outcomes is needed in this cancer population. We developed an objective tool of chemotherapy therapy that was not subject to observer bias.
In recent years, consumer-grade biometric sensors have been introduced as tools with which to objectively assess PS, thereby bypassing provider bias and patient-physician discordance.17 Wearable sensors, such as the Apple Watch, FitBit, Microsoft Band, and Microsoft Kinect, can systematically capture and quantify a patient’s physical activity. We previously showed that Microsoft Kinect has a high concordance with physician-assessed PS and, more recently, that activity measured by the Microsoft Band corresponds with unexpected health care encounters (UHE).18,19 In this observational study, we sought to further elucidate the Microsoft Kinect’s predictability of low physical activity (LPA) and UHE in patients with cancer receiving highly emetogenic chemotherapy. We hypothesized that poor movements captured by noninvasive motion-capture systems in the clinic can identify patients who are at risk of complications, such as UHEs and LPA, while receiving highly emetogenic chemotherapy.
METHODS
Trial Design
This study was a multicenter, single-arm, observational trial conducted in the United States between July 2016 and July 2017. Sites included the Norris Comprehensive Cancer Center, the Los Angeles County and University of Southern California Medical Center, and the MD Anderson Cancer Center. The study was designed to compare kinematic findings obtained from motion-capture systems (eg, Microsoft Kinect) and wearable motion sensors (eg, Microsoft Band 2) to determine if in-clinic movements correlate with UHEs and physical activity at home. The institutional review boards at all participating sites approved the study protocol. Written informed consent was obtained from all participants.
Participants
Eligibility criteria included age older than 18 years, diagnosis of a solid tumor, anticipated treatment of two cycles of highly emetogenic chemotherapy,20 the ability to autonomously ambulate without an assistive device, and completion of two separate kinematic evaluations.
Clinical Exercises and Motion Capture
Patients underwent two clinically supervised tasks, including chair-to-table (CTT) and get-up-and-walk (GUP) tasks. The CTT task begins with patients standing up from a chair while rotating the hip and left leg and pivoting on the right leg. The task design requires a large range of motion from the left lower extremities. The GUP task requires patients to stand up and walk to a marker 8 feet away, turn around, and walk back to the starting position. We analyzed the entire CTT task and the walking portion of GUP using the motion-capture system.
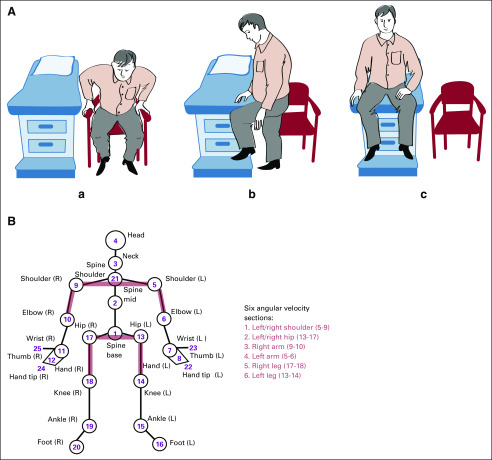
The two tasks were performed pretreatment (visit-1) and post-treatment (visit-2) with a median 21-day gap between visits (mean, 24.5 days). We used Microsoft Kinect, a depth-sensing motion-capture camera, to record the exercises, and three-dimensional positions of 25 anatomic sites were extracted (Fig 1). Subsequently, six types of kinematic features were calculated: velocity, acceleration, specific kinetic energy, specific potential energy, sagittal angle, and angular velocity (Appendix). We excluded wrist, hand, ankle, and foot joints (Fig 1) from the statistical analysis as the motion-capture signal for these joints is less reliable. The combination of selected joints and kinematic features capture the underlying biomechanics of patient movement and are therefore selected for interpatient comparison.
FIG 1.
(A) Illustration of the chair-to-table exam maneuver demonstrating (a) sitting at rest, (b) standing, rotating hip, pivoting on right leg, and using the left (nonpivoting) leg to move onto exam table, (c) sitting on exam table. (B) Schematic of anatomic sites detected in motion capture, along with 6 two-joint sections (red) whose angular velocities are extracted from raw kinematic data. L, left; R, right.
Physical Activity Measurement
Patient outcomes were grouped by activity level and UHEs. Patients’ overall physical activity was tracked via the Microsoft Band 2 wrist motion sensor for a 10-hour period daily over a 60-day study period while receiving chemotherapy. Using energy expenditure data from the band, we identified patients with low and high physical activity, defined as nonsedentary physical activity (NSPA) for 20 hours or less (LPA positive) and > 20 hours (LPA negative) over a course of 60 days, respectively. NSPA was defined as the hours the patient exhibited more than 1.5 metabolic equivalent of task, where metabolic equivalents of task themselves were estimated by dividing the band hourly energy expenditure by daily reported weight. The 20-hour cutoff is used to balance the need for two similar-sized, yet differentiable, patient groups in the analysis. Details of NSPA and LPA calculations are in the Appendix.
Similarly, treatment-associated complications were measured by UHE as defined by unplanned encounters at triage/infusion centers, clinics, hospitals or the emergency room during the 60-day study period. Scheduled hospital admissions, infusions, and clinic appointments were not included.
Statistical Analysis
We differentiated patients by the average of visit-1 and visit-2 statistics for the set of kinematic features and correlated to two binarized clinical outcomes UHE and LPA. Welch t test was used to test whether the averaged statistics are different for positive and negative groups, thereby revealing kinematic features that distinguish between UHE-positive and UHE-negative patients, and similarly LPA-positive and LPA-negative patients. Welch t test, also known as the unequal variance t test, allows the central tendency of two groups of unequal sizes and unequal variance to be tested for equivalence.21 We built logistic regression classifiers of UHE and LPA from each feature and quantified their ability to separate patients into risk groups using the area under the curve (AUC) of the mean receiver operating characteristic (ROC) curve from three-fold stratified cross-validation.
Ethics Approval and Consent
All procedures involving human participants were conducted in accordance with the standards of both the University of Southern California and MD Anderson Cancer Center Institutional Review Boards and the 1964 Declaration of Helsinki. Informed consent was obtained from all participants.
RESULTS
Patient Enrollment Criteria
Of the 60 patients who were screened and amenable to participating in the study, 38 patients (20 female) completed the study without dropout and had associated UHEs and physical activity results (Fig 2). The mean age of participants was 48.3 years. Predominant tumor types were breast, testicular, and head and neck cancer (Table 1). Chemotherapy was primarily of curative intent for most patients. Patient demographics are listed in Table 1. Of the initial 60 patients, 15 did not enroll, as they did not meet eligibility criteria. Of the 45 patients who enrolled in the study, seven did not complete the study given clinical deterioration or technologic hurdles (Fig 2).
FIG 2.
Flowchart showing the number of patients who were initially screened, started on trial, and completed the trial.
TABLE 1.
Baseline Characteristics of Participants

Outcomes
Eighteen patients had no UHEs (UHE negative) and 20 patients had at least 1 UHE (UHE positive). Physical activity data were collected for all 38 patients by monitoring via a wearable motion sensor. Twenty-four patients demonstrated low activity levels (LPA positive) and 14 demonstrated high activity levels (LPA negative). Of UHE-positive patients, 75% were also LPA positive, and 63% of LPA-positive patients were also UHE positive. Of LPA-negative patients, 64% were also UHE negative; however, only 50% of UHE-negative patients were also LPA negative.
UHEs
We report the kinematic features that correlate most with UHEs according to Welch t test (Table 2) and ROC analysis (Table 3). CTT kinematic features have higher t test scores and AUC for UHE than GUP features. Figure 3A shows the ROC curves for the features with the highest AUC values for UHE. Hip, spine base, and left (pivoting) knee kinematics from CTT form the best classifiers of UHE. Mean accelerations during CTT of the left knee, left hip, and spine base were found to be higher in patients with no UHE compared with those with at least one encounter (Table 2). The full list of 29 features with significant t test scores (P < .05) are listed in the Appendix (Appendix Figs A1-A3 and Appendix Table A1). Age (t = 0.1617; P = .872) and percent change in weight across pre- and post-treatment (t = −0.502; P = .619) did not produce statistically significant t tests for UHE and had low AUC values (0.212 ± 0.093 and 0.521 ± 0.0745 for age and percent weight change, respectively).
TABLE 2.
Test for Difference in Mean Values of Kinematic Features by UHE and LPA
TABLE 3.
Logistic Regression AUC Values of Single-Feature Classifiers of UHE and LPA
FIG 3.
Kinematic features that differentiate patients with zero unexpected hospital encounters (UHEs) from patients with one or more hospitalizations. (A) Receiver operating characteristic curves for features with the highest areas under the curve (AUC). (B) Box plots for features with high t test scores (UHE negative, blue; UHE positive, orange). acc, acceleration (m/s2); av-hz, angular velocity about horizontal axes (radians/s); CTT, chair to table; GUP, get up and walk; pe, specific potential energy (J/kg).
Physical Activity
We report kinematic features that correlate most with physical activity according to Welch t test (Table 2) and ROC analysis (Table 3). Unlike UHE, both CTT and GUP features are in the list of LPA-differentiating features. Angular velocities, particularly those of the legs, differentiate activity groups the most. Although fewer kinematic features from the clinical exercises are significantly and strongly correlated with LPA versus UHE according to t test scores (Table 2), the average AUC of the top 15 features is higher for LPA (mean AUC, 0.751) than for UHE (mean AUC, 0.723). The full list of 19 features with significant t test scores (P < .10) are listed in the Appendix (Appendix Figs A4 and A5 and Appendix Table A2).
Figure 4 depicts kinematic features associated with LPA. Figure 4A shows ROC curves for the features with the highest AUC values for LPA where the mean right leg angular velocity about the horizontal during CTT forms the best classifier of LPA (AUC, 0.830 ± 0.08). Left-arm mean angular velocities during GUP are greater (absolute value) for higher activity patients, as seen in Figure 4B.
FIG 4.
Top three kinematic features that differentiate patients with low and high nonsedentary physical activity. (A) Receiver operating characteristic curves for features with the highest area under the curve (AUC). (B) Box plots for features with high t test scores (low physical activity [LPA] negative, blue; LPA positive, orange). acc, acceleration (m/s2); av-hz, angular velocity about horizontal axes (radians/s); av-vt, angular velocity about vertical axis (radians/s); CTT, chair to table; GUP, get up and walk.
DISCUSSION
This study examined the utility of a consumer-grade motion-capture system, the Microsoft Kinect, in identifying patients with cancer undergoing chemotherapeutic treatment who are at risk for treatment complications, as measured by UHE. Activity level, along with the risk of therapy-associated complications, could be predicted on the basis of movement within the office. These findings should not be surprising to most clinicians, who are usually aware that people who appear to be frail are more likely to have difficulty when receiving chemotherapy. What may be interesting to these same clinicians is that the visualization of frailty can be captured, recorded, and measured using noninvasive technology. Although activity levels outside the clinic may vary dramatically, especially over the 60-day study period, and the two clinical video samples per patient are brief, we found them to be descriptive of activity levels in the free environment.
Our findings expand on the investigation of widely available, consumer-grade biometric sensors as emerging technologic replacements of the conventional PS. It has been demonstrated previously that wearable activity monitors are feasible tools for measuring activity outside of the clinical setting.22 The Actigraph, a triaxial accelerometer, has been shown to be of value in determining quality of life and physical activity, and has even been found to be superior to ECOG PS in predicting 6-month survival.23 More recently, we demonstrated high correspondence between Actigraph-generated steps and patient-reported outcomes, such as physical function and fatigue.24 Trials have also shown that activity levels, as measured by another wearable modality, the Fitbit, predict adverse events and 6-month mortality in patients with cancer.25 Indeed, in the past decade, the number of oncology trials investigating the feasibility of wearable sensors has quadrupled, and their application to drug development and cancer care is a point of great interest and promise.26,27 Most of these studies focus on activity levels outside of the clinical setting. Our study is unique in that it aims to use a motion-capture system to predict adverse outcomes during an oncology clinic visit, the setting in which oncologists identify patients who are candidates for newly emerging therapies or who are able to participate in clinical trials.
The clinical application of a motion-sensor system has multiple implications for how oncologists identify patients who are able to tolerate novel therapies and those who are at risk for drug toxicity. Physical activity itself plays a key role in improved outcomes.25 In addition, approximately 67% of hospital encounters in the first year after cancer diagnosis are unexpected.28 UHEs may cause interruptions and delays in therapy.
Motion-capture systems are already transforming the study of cancer care, and ways to integrate the technology into current clinical trials and drug development have been proposed.29 For example, motion-capture systems have the potential to add a degree of objectivity to the clinical trial landscape, particularly in the area of adverse event attribution. Currently, adverse event attribution of a study drug is divided into a 5-tier system, spanning from not related to definitely related. It has been demonstrated that the majority of clinicians do not have formal training in attribution and that the process is frequently completed without full data.30,31 The system may lend to the misattribution of excess toxicity to a drug administered to a human participant with poorer PS, and thus less tolerability, than deemed by a clinician observer during enrollment.
Indeed, the need for standard operating procedures for collecting baseline data has recently been proposed.32 In-clinic motion-capture systems would enable standardization of PS measurement and provide a means by which to capture and record PS so that all human participants across multiple centers can be accurately assessed throughout the course of a clinical trial by investigators, regardless of study role and geographic location. This concept also transcends patients and clinicians to also provide objective and transparent patient-specific data to all stakeholders in the approval of a drug, including pharmaceutical companies and insurance companies.
Although we foresee the use of motion-capture systems as a means to optimize the integrity and accuracy of clinical trials, we also caution against the use of motion-capture systems in further limiting patient eligibility for clinical trials. As previously mentioned, RCTs often use PS as an inclusion criterion; therefore, the precise delineation of PS could place many patients in lower-performing categories than they would have been by physicians, thereby excluding them from trial eligibility. We would view this as a misuse of the technology. Instead, we propose that it be used as a means with which to stratify trial results by performance groups and enhance the applicability of trial findings to real-world populations. In doing so, we are hopeful that motion-capture systems serve as tools that forward the mission of the Institute of Medicine and ASCO to modernize and broaden clinical trial eligibility criteria.33
Several limitations of our study must be noted. We observed a small sample size and had an observational study design, rather than a RCT design. Given the small size of this study, as well as the heterogeneous population, we did not perform an adjusted analysis of our results. A future validation study with a more homogenous population would allow for analysis of potential confounders. With regard to the Microsoft Band 2 wearable sensor, we do not have data on 24-hour patient compliance and syncing habits. Of the initial 60 patients who were screened, 22 did not ultimately complete the study, largely as a result of having more advanced disease and receiving palliative chemotherapy. Our results, therefore, may not best reflect the utility of a motion-capture system in patients with more advanced cancer. Moreover, our trial also included only patients with solid tumors receiving high emetogenic chemotherapy; therefore, the data may not be extractable to patients with hematologic malignancies or those receiving other types of anticancer treatments, including immunotherapy, or combination therapy with surgery and radiation.
In summary, our findings suggest the Microsoft Kinect to be one tool with which oncologists can make more precise assessments of treatment tolerability in the clinical setting, thus improving patient treatment options and outcomes and also refining the means by which oncologic drug trials are conducted. Future directions include designing studies investigating multiple types of motion-capture systems. Although wearable monitors have thus far been shown to be of utility in monitoring the activity levels of patients with cancer, the advent and further study of in-clinic motion-capture systems may obliterate the need for continuous monitoring systems. We also aim to study a large population across multiple centers, stratified by cancer type and therapy type. Additional larger-scale and more homogenous studies may validate our results and also potentially clarify how motion-capture systems can be used in conjunction with wearable sensors. Furthermore, we hope future studies identify in-clinic kinematics associated with specific malignancy complications, such as infection and venous thromboembolism. Identification of movements that relate to common, tangible oncologic complications can provide oncologists with specific tools for prevention and early treatment.
Appendix
Kinetic Feature Extraction
Details of kinematic feature extraction from the raw three-dimensional position motion-capture data are described here. Anatomic site position vectors are 3-dimensional time series constructed from position at each time point ri(t) = [xi(t), yi(t), zi(t)] for i = 25 anatomic sites. The position vectors are used to calculate velocity magnitude and acceleration magnitudept of each anatomic site using the mean-value theorem where a single dot superscript designates a derivative with respect to time and a double dot superscript is a second derivative with respect to time. As a result of the lack of distribution of mass information, specific kinetic energy and specific potential energy . We define sagittal angle as the angle formed between , the vector originating at the spine base and pointing in the direction of motion, and , the vector connecting anatomic site 1 (spine base) and 3 (neck) at each time point. The angular velocity of the sections defined in Figure 1 are calculated using 3-dimensional rigid body kinematic equations for relative motion.
Sagittal Angle Calculation
We define sagittal angle as the angle formed between , the vector originating at the spine base and pointing in the direction of motion, and , the vector connecting anatomic site 1 (spine base) and 3 (neck) at each time point. The sagittal angle is calculated using the inverse tangent of the ratio of the cross product and dot product of and , .
Angular Velocity Calculation
The angular velocity of the sections defined in Figure 1 are calculated using 3-dimensional rigid body kinematic equations for relative motion. A section (Fig 1) is treated as a rigid bar and is defined by two anatomic points—for example, left and right hips define the hip section—and we refer generically to these two ends as point A and point B. We calculate the velocities of these two points from the position vectors using the mean-value theorem, as mentioned previously. Therefore, using these two velocities, the angular velocity of the section can be isolated in the relative velocity vector equation, , where is the vector from point A to point B . This vector equation has 3 components corresponding to the three directions and requires an additional equation to solve for the 3 components of the angular velocity. Consequently, we use a kinematic restriction equation , because the angular motion of the section along the axis of the section does not affect its action. This allows for a solution to the three components of the angular velocity vector :
These equations are solved at each time point to get the time series of angular velocities for each section in Figure 1.
Microsoft Band 2
Microsoft Band 2 is a wrist-worn activity tracker containing multiple sensors that provide hourly activity measurements, including energy expenditure, step count, and heart rate. To allow activity level comparison across patients, we estimated hourly metabolic equivalent of task (MET), where 1 MET corresponds to a person’s basal metabolism. METs were estimated by dividing band hourly energy expenditure by daily reported weight to account for weight changes. Baseline activity level was corrected to 1 MET using line fit to normalize METs across patients. We computed the nonsedentary physical activity hours for the compliance period, as the number of hours a patient exhibited > 1.5 METs. Of note, as band data were available on an hourly interval, a 1.5-MET threshold corresponds to an energy expenditure of 1.5 METs on average for the entire hour.
We defined daily band wear compliance as the study participant wearing the band for 8 hours or longer during the 10 AM to 8 PM period. This period was selected in accordance with this cohort’s expected common waking hours. We defined overall band wear compliance as the study participant being daily band wear compliant for 80% or more of days during the 60-day band wear period. For the analysis, we considered only band data for the 10 AM to 8 PM compliance period.
Unexpected Hospital Encounters
Two-sample t tests were performed to determine if mean values of kinematic features are different for patients with zero unexpected hospital encounters (UHEs; UHE negative) and patients with one or more UHE (UHE positive), and the distribution of the resulting t test scores and significance values for the entire set of 526 features is shown in Appendix Figure A1. The full list of 29 significant (P < .05) t test scores is shown in Appendix Table A1, and box plots of these significantly differentiating kinematic features are shown in Appendix Figures A2 and A3.
Low Physical Activity
Two-sample t tests were performed to determine if mean values of kinematic features are different for patients with more than 20 hours of nonsedentary physical activity (low physical activity [LPA] negative) compared with patients with 20 hours or less of nonsedentary physical activity (LPA positive), and the distribution of the resulting t test scores and significance values for the entire set of 526 features is shown in Appendix Figure A4. A full list of 19 significant (P < .10) t test scores is shown in Appendix Table A2, and box plots of these significantly differentiating kinematic features are shown in Appendix Figure A5.
FIG A1.
Distribution of t test scores and significance values from 2-sample t tests for differences in mean values of kinematic features between patients with no unexpected hospital encounters (UHEs; UHE = 0) and patients with one or more unexpected hospitalizations (UHE = 1).
FIG A2.
Box plots of kinematic features that significantly differentiate unexpected hospital encounter (UHE) –negative (blue) and UHE-positive (orange) patients. Kinematic features 1-20. acc, acceleration (m/s2); av-hz, angular velocity about horizontal axes (radians/s); CTT, chair to table; GUP, get up and walk; ke, specific kinetic energy (J/kg); pe, specific potential energy (J/kg); UHE, unexpected health encounter; vel, velocity (m/s).
FIG A3.
Box plots of kinematic features which significantly differentiate unexpected hospital encounter (UHE) –negative (blue) and UHE-positive (orange) patients. Kinematic features 21-29. acc, acceleration (m/s2); av-hz, angular velocity about horizontal axes (radians/s); CTT, chair to table; ke, specific kinetic energy (J/kg); pe, specific potential energy (J/kg); UHE, unexpected health encounter.
FIG A4.
Distribution of t test scores and significance values from 2-sample t tests for differences in mean values of kinematic features between unexpected hospital encounter (UHE) –negative and UHE-positive patients. LPA, low physical activity.
FIG A5.
Box plots of kinematic features that significantly differentiate between low physical activity (LPA) –negative (blue) patients and LPA-positive (orange) patients. Kinematic features 1-19. acc, acceleration (m/s2); av-hz, angular velocity about horizontal axes (radians/s); av-tot, the total angular velocity (radians/s); av-vt, angular velocity about vertical axis (radians/s); CTT, chair to table; GUP, get up and walk; ke, specific kinetic energy (J/kg); pe, specific potential energy (J/kg); vel, velocity (m/s).
TABLE A1.
Full List of Kinematic Features That Significantly (P < .05) Differentiate Between UHE-Negative and UHE-Positive Patients
TABLE A2.
Full List of Kinematic Features (feature 1-5: P < .05; feature 6-22: .05 < P < .10) That Differentiate Between LPA-Negative and LPA-Positive Patients
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
SUPPORT
Supported in part by National Cancer Institute Grant No. P30CA014089 and by Noetic Contract FFP 012016 (USA 051) Collaborative Initiative on Human Performance Optimization, National Cancer Institute Grant No. P30CA016672, an MD Anderson Cancer Center Support Grant, and a State of Texas Rare and Aggressive Breast Cancer Research Program Grant.
AUTHOR CONTRIBUTIONS
Conception and design: Zaki Hasnain, Cyrus Shahabi, Frankie A. Cozzens Philips, Jerry S.H. Lee, Sean E. Hanlon, Paul K. Newton, Peter Kuhn, Jorge Nieva
Financial support: Frankie A. Cozzens Philips, Paul K. Newton, Peter Kuhn, Jorge Nieva
Administrative support: Sriram Yennu, Peter Kuhn, Jorge Nieva
Provision of study materials or patients: Naoto T. Ueno, Sriram Yennu, Peter Kuhn, Jorge Nieva
Collection and assembly of data: Zaki Hasnain, Tanachat Nilanon, Ming Li, Aaron Mejia, Anand Kolatkar, Luciano Nocera, Naoto T. Ueno, Sriram Yennu, Peter Kuhn, Jorge Nieva
Data analysis and interpretation: Zaki Hasnain, Tanachat Nilanon, Anand Kolatkar, Luciano Nocera, Cyrus Shahabi, Jerry S.H. Lee, Poorva Vaidya, Naoto T. Ueno, Sriram Yennu, Paul K. Newton, Peter Kuhn, Jorge Nieva
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Anand Kolatkar
Stock and Other Ownership Interests: Epic Sciences
Patents, Royalties, Other Intellectual Property: High-definition single-cell assay technology was developed by myself and members of my team while at The Scripps Research Institute, which has subsequently licensed the technology to Epic Sciences for exclusive commercial development
Luciano Nocera
Patents, Royalties, Other Intellectual Property: Application No. 62/825,965: System and method for determining quantitative health-related performance status of a patient (Inst)
Naoto T. Ueno
Honoraria: Kyowa Hakko Kirin, Roche, Amgen, Chugai, Henry Steward Talks, Taiho Pharmaceutical, Eisai
Consulting or Advisory Role: Samsung Bioepis, Daiichi Sankyo, Immunomedics
Research Funding: Medivation, Bayer, Amgen, Puma Biotechnology, Merck, Daiichi Sankyo, Celgene, GlaxoSmithKline, Kyowa Hakko Kirin, Bio-Path Holdings, Novartis, Sysmex
Travel, Accommodations, Expenses: Kyowa Hakko Kirin
Sriram Yennu
Consulting or Advisory Role: Pfizer
Research Funding: Bayer (Inst), Genentech (Inst), Helsinn Therapeutics (Inst)
Peter Kuhn
Stock and Other Ownership Interests: Epic Sciences
Consulting or Advisory Role: Epic Sciences
Research Funding: Gilead Sciences, Kite Pharma, Amgen, AbbVie, Daiichi Sankyo, Eli Lilly, Novartis, Fluidigm
Patents, Royalties, Other Intellectual Property: High-definition single-cell assay technology was developed by myself and members of my team while at The Scripps Research Institute, which has subsequently licensed the technology to Epic Sciences for exclusive commercial development
Expert Testimony: BDMK Law
Jorge Nieva
Stock and Other Ownership Interests: Epic Sciences, Cansera
Consulting or Advisory Role: AstraZeneca, Western Oncolytics, Fujirebio Diagnostics, Takeda
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: Patent pending on movement and unexpected health care encounters
No other potential conflicts of interest were reported.
REFERENCES
- 1.Chow R, Chiu N, Bruera E, et al. Inter-rater reliability in performance status assessment among health care professionals: A systematic review. Ann Palliat Med. 2016;5:83–92. doi: 10.21037/apm.2016.03.02. [DOI] [PubMed] [Google Scholar]
- 2.Ando M, Ando Y, Hasegawa Y, et al. Prognostic value of performance status assessed by patients themselves, nurses, and oncologists in advanced non-small cell lung cancer. Br J Cancer. 2001;85:1634–1639. doi: 10.1054/bjoc.2001.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popovic G, Harhara T, Pope A, et al. Patient-reported functional status in outpatients with advanced cancer: Correlation with physician-reported scores and survival. J Pain Symptom Manage. 2018;55:1500–1508. doi: 10.1016/j.jpainsymman.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Schnadig ID, Fromme EK, Loprinzi CL, et al. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer. 2008;113:2205–2214. doi: 10.1002/cncr.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosolem MM, Rabello LSCF, Lisboa T, et al. Critically ill patients with cancer and sepsis: Clinical course and prognostic factors. J Crit Care. 2012;27:301–307. doi: 10.1016/j.jcrc.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T, Shimokawa M, Matsuo K, et al. Risk factors for delayed chemotherapy-induced nausea and vomiting with low-emetic-risk chemotherapy: A prospective, observational, multicenter study. Cancer Manag Res. 2018;10:4249–4255. doi: 10.2147/CMAR.S176574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778–784. doi: 10.1001/jamaoncol.2015.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 9.Cheng S, Qureshi M, Pullenayegum E, et al. Do patients with reduced or excellent performance status derive the same clinical benefit from novel systemic cancer therapies? A systematic review and meta-analysis. ESMO Open. 2017;2:e000225. doi: 10.1136/esmoopen-2017-000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sargent DJ, Köhne CH, Sanoff HK, et al. Pooled safety and efficacy analysis examining the effect of performance status on outcomes in nine first-line treatment trials using individual data from patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:1948–1955. doi: 10.1200/JCO.2008.20.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Köhne C-H, Cunningham D, Di Costanzo F, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: Results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13:308–317. doi: 10.1093/annonc/mdf034. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney CJ, Zhu J, Sandler AB, et al. Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: A phase II trial in patients with metastatic nonsmall cell lung carcinoma. Cancer. 2001;92:2639–2647. doi: 10.1002/1097-0142(20011115)92:10<2639::aid-cncr1617>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Donia M, Kimper-Karl ML, Høyer KL, et al. The majority of patients with metastatic melanoma are not represented in pivotal phase III immunotherapy trials. Eur J Cancer. 2017;74:89–95. doi: 10.1016/j.ejca.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Wong A, Williams M, Milne D, et al. Clinical and palliative care outcomes for patients of poor performance status treated with antiprogrammed death-1 monoclonal antibodies for advanced melanoma. Asia Pac J Clin Oncol. 2017;13:385–390. doi: 10.1111/ajco.12702. [DOI] [PubMed] [Google Scholar]
- 15.Ommundsen N, Wyller TB, Nesbakken A, et al. Frailty is an independent predictor of survival in older patients with colorectal cancer. Oncologist. 2014;19:1268–1275. doi: 10.1634/theoncologist.2014-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huisingh-Scheetz M, Walston J. How should older adults with cancer be evaluated for frailty? J Geriatr Oncol. 2017;8:8–15. doi: 10.1016/j.jgo.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly CM, Shahrokni A. Moving beyond Karnofsky and ECOG performance status assessments with new technologies. J Oncol. 2016;2016:6186543. doi: 10.1155/2016/6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasnain Z, Li M, Dorff T, et al. Low-dimensional dynamical characterization of human performance of cancer patients using motion data. Clin Biomech (Bristol, Avon) 2018;56:61–69. doi: 10.1016/j.clinbiomech.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilanon T, Nocera LP, Martin AS, et al. Use of wearable activity trackers in predicting unexpected healthcare encounters in cancer patients undergoing chemotherapy. JCO Clin Cancer Inform. doi: 10.1200/CCI.20.00023. (submtted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oncology Nursing Society Emetogenic risk of chemotherapy and biotherapy agents. https://www.ons.org/sites/default/files/emetogenicity_tool.pdf
- 21.Ruxton GD. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Behav Ecol. 2006;17:688–690. [Google Scholar]
- 22.Gupta A, Stewart T, Bhulani N, et al. Feasibility of wearable physical activity monitors in patients with cancer. JCO Clin Cancer Inform. doi: 10.1200/CCI.17.00152. 10.1200/CCI.17.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujisawa D, Temel JS, Greer JA, et al. Actigraphy as an assessment of performance status in patients with advanced lung cancer. Palliat Support Care. 2019;17:574–578. doi: 10.1017/S1478951518001074. [DOI] [PubMed] [Google Scholar]
- 24.Broderick JE, May M, Schwartz JE, et al. Patient reported outcomes can improve performance status assessment: A pilot study. J Patient Rep Outcomes. 2019;3:41. doi: 10.1186/s41687-019-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gresham G, Hendifar AE, Spiegel B, et al. Wearable activity monitors to assess performance status and predict clinical outcomes in advanced cancer patients. NPJ Digit Med. 2018;1:27. doi: 10.1038/s41746-018-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gresham G, Schrack J, Gresham LM, et al. Wearable activity monitors in oncology trials: Current use of an emerging technology. Contemp Clin Trials. 2018;64:13–21. doi: 10.1016/j.cct.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox SM, Lane A, Volchenboum SL. Use of wearable, mobile, and sensor technology in cancer clinical trials. JCO Clin Cancer Inform. doi: 10.1200/CCI.17.00147. 10.1200/CCI.17.00147. [DOI] [PubMed] [Google Scholar]
- 28.Whitney RL, Bell JF, Tancredi DJ, et al. Unplanned hospitalization among individuals with cancer in the year after diagnosis. J Oncol Pract. 2019;15:e20–e29. doi: 10.1200/JOP.18.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao Y, Thompson C, Peterson S, et al. Am Soc Clin Oncol Educ B. 2019. The future of wearable technologies and remote monitoring in health care; pp. 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le-Rademacher JG, Storrick EM, Jatoi A, et al. Physician-reported experience and understanding of adverse event attribution in cancer clinical trials. Mayo Clin Proc Innov Qual Outcomes. 2019;3:176–182. doi: 10.1016/j.mayocpiqo.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee SD, Coombes ME, Levine M, et al. A qualitative study evaluating causality attribution for serious adverse events during early phase oncology clinical trials. Invest New Drugs. 2011;29:1013–1020. doi: 10.1007/s10637-010-9456-9. [DOI] [PubMed] [Google Scholar]
- 32.George GC, Barata PC, Campbell A, et al. Improving attribution of adverse events in oncology clinical trials. Cancer Treat Rev. 2019;76:33–40. doi: 10.1016/j.ctrv.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Jin S, Pazdur R, Sridhara R. Re-evaluating eligibility criteria for oncology clinical trials: Analysis of investigational new drug applications in 2015. J Clin Oncol. 2017;35:3745–3752. doi: 10.1200/JCO.2017.73.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]