Abstract
PURPOSE
While cancer survival is improving across most developed nations, those improvements are not shared equally within their population. Using high-quality national data, we have reviewed the extent to which cancer survival inequities are persisting for indigenous Māori compared with non-Māori New Zealanders and the extent to which these disparities are driven by deprivation, comorbidity, and stage of disease.
METHODS
Incident cases of cancer (2007-2016) were extracted from the New Zealand Cancer Registry and linked to mortality and hospitalization data. Descriptive, Kaplan-Meier, and Cox regression methods were used to compare survival outcomes between Māori and non-Māori.
RESULTS
Māori continue to have poorer survival than non-Māori for 23 of the 24 most common causes of Māori cancer death, with the extent of this disparity ranging from 12% to 156%. The magnitude of these disparities varies according to deprivation, comorbidity, and stage. Of note, there was a tendency for survival disparities to be largest among those with no comorbidity.
CONCLUSION
Māori continue to experience substantial cancer survival inequities. These observations are in keeping with reports from previous decades, which suggest that these disparities persist despite heightened attention. Reduction of the cancer burden on Māori and achievement of equitable survival outcomes require us to prevent cancer for Māori where we can, diagnose Māori patients early when we cannot, and once diagnosed, deliver equitable care to Māori patients at each step along the treatment path.
INTRODUCTION
Overall, cancer survival is improving in high-income countries.1 The factors that underlie these improvements vary between cancer types but include improvements in access to evidence-based screening and early detection and advances in treatment and supportive care.1 However, these improvements are not equally distributed within populations, with evidence demonstrating that access to early diagnosis and best practice treatment varies according to ethnicity and socioeconomic status2 and has a subsequent impact on cancer survival.3-6 Other factors such as where a patient lives in relation to health care services,7,8 older age, and comorbidity can affect the likelihood of a patient receiving best practice cancer care and, in turn, worsen survival outcomes.9
CONTEXT
Key Objective
There has been an increased focus over the past decade on identifying and addressing cancer survival inequities between indigenous Māori and non-Māori New Zealanders. But has this focus led to any meaningful change in these inequities over this time period, and what can we do to eliminate them?
Knowledge Generated
Despite heightened attention, Māori continue to have poorer survival for 23 of the 24 measured cancers. By examining how these survival disparities differ by factors such as comorbidity and stage of disease, we discuss how the observed inequities can be eliminated for the most common cancers to affect Māori.
Relevance
Using high-quality national data sets, we have described survival inequities experienced by Māori, considered the role of factors such as comorbidity as drivers of these inequities, and provided cancer-specific actions to eliminate them. Themes addressed in this article are likely have application to other indigenous populations worldwide.
In New Zealand, there is evidence of persisting survival disparities between the indigenous Māori population and our non-Māori population. In a report covering 1996-2006, Māori had substantially poorer cancer-specific survival than non-Māori for 18 of the 22 measured cancer sites, with Māori 24%-131% more likely to die once diagnosed than non-Māori, depending on the cancer.10 This observation of poorer cancer survival for Māori has been repeated in several cancer-specific studies in New Zealand.11-15 During the past decade, there has been an increased focus on identifying and addressing the causes of these inequities. In this article, we use the most recent available data to review the extent to which cancer survival inequities persist for Maori compared with non-Māori as well as the extent to which these disparities are driven by socioeconomic status, patient comorbidity, and stage of disease at diagnosis for the purpose of guiding actions to reduce these inequities.
METHODS
Participants and Data Sources
Incident cases of cancer that occurred between 2007 and 2016 were derived from the New Zealand Cancer Registry (NZCR). The NZCR is a nationally mandated population-based registry of all malignancies diagnosed in New Zealand, with the exception of basal and squamous cell skin cancers.16 All cancer sites were included in the combined cancer analysis (N = 215,783 patients; 21,066 Māori, 194,717 non-Māori), whereas for other analyses, we focused only on the most common causes of cancer death for Māori. Only malignant cancers were included in the analysis (ie, no in situ cancers were included).
These cases were linked through encrypted National Health Index number to the national mortality collection, which records all deaths that occur in New Zealand as well as their underlying cause. Cause of death is attributed by Ministry of Health staff by combining information from medical certificates of cause of death, coroners’ reports from Coronial Services, hospital discharge data (both public and private), the NZCR, the New Zealand Transport Agency, Water Safety New Zealand, and direct information gathered from certifying physicians, coroners, and medical records officers in public hospitals.17 Ethical approval for this study was sought and received from the University of Otago Human Ethics Committee (reference # HD18/056).
Variables
Age at diagnosis was defined by subtracting the date of cancer diagnosis as recorded in the NZCR from the individual’s date of birth (also recorded in the NZCR). Sex was derived from the NZCR and defined as either female or male. Prioritized ethnicity was derived from the NZCR and defined for this study as Māori or non-Māori. Europeans comprised the majority of the non-Māori group (89%), followed by Pacific (4%), Asian (4%), and Middle Eastern-Latin American-African/other New Zealanders (3%).
Level of socioeconomic deprivation was defined using the NZDep deprivation scale. NZDep is a small area-based deprivation index that uses multiple variables to define the level of deprivation of a given area.18 Missing data prevented the attribution of deprivation for 9,435 patients with cancer (4% of the cohort).
Patient comorbidity was defined using the C3 Index, a cancer-specific measure of patient comorbidity that uses public and private inpatient hospitalization data (National Minimum Dataset) to define the presence or absence of 42 individual conditions.19 All International Classification of Diseases, 10th Revision–coded diagnoses recorded in the 5 years before the date of cancer diagnosis were used to calculate a C3 Index score for each patient, with each condition weighted according to its relationship with noncancer mortality in a cancer population.19 Condition weights were then summed to give the final C3 Index score, categorized as 0 (score ≤ 0), 1 (≤ 1), 2 (≤ 2), and 3 (> 2). Those with none of the included conditions over the lookback period were assigned a score of 0.
Cancer stage at diagnosis was determined from the NZCR and based on the SEER Summary Stage method (A-F).20 Stage was categorized into local (B), regional (C and D), advanced (E), and unstaged (F).21 The degree to which cancers were unstaged varied among cancers (eg, lung 65%, stomach 44%, breast 11%, pancreatic 36%, colon 11%, liver 65%), and in nearly all cases, there was no difference between Māori and non-Māori in terms of the proportion unstaged (eg, lung: Māori 38%, non-Māori 36%; stomach: Māori 33%, non-Māori 46%; breast: Māori 11%, non-Māori 11%; pancreatic: Māori 35%, non-Māori 36%; colon: Māori 12%, non-Māori 11%; liver: Māori 64%, non-Māori 65%).
Statistical Analysis
Descriptive analysis was conducted to describe the total number of cancer-specific deaths that occurred among Māori and non-Māori patients with cancer. The maximum length of follow-up time was 10 years. Crude Kaplan-Meier analysis was conducted to extract 1-, 3-, and 5-year cancer-specific survival for Māori and non-Māori for all cancers (single analysis) and by cancer type (multiple analyses).
Cox proportional hazards models were used to describe the extent to which Māori were more (or less) likely to die as a result of their cancer than non-Māori, adjusted for age (continuous variable) and sex (categorical variable) where relevant. These results were described using hazard ratios (HRs), with non-Māori as the reference group. In addition, we calculated excess cancer mortality as the percentage that the HR was > 1 or < 1 (eg, an HR of 1.20 equates to 20% excess mortality). Finally, a series of additional Cox models were conducted that compared Māori and non-Māori mortality separately stratified by each category or level of deprivation, comorbidity, and stage. Patients were censored at the end of follow-up (December 31, 2016) or on date of death if the listed underlying cause of death was not their primary cancer. All analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC) and Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA) software.
RESULTS
The number of deaths among Māori and non-Māori patients with cancer is shown in the Data Supplement for the top 24 causes of cancer death for Māori along with 1-, 3-, and 5-year cumulative survival percentages. Lung cancer had the highest mortality burden for Māori, with > 300 Māori (diagnosed between 2007 and 2016) dying per year and only 10% surviving to 5 years postdiagnosis (compared with 15% of non-Māori). The next 5 cancers in order of mortality burden for Māori were stomach (47 Māori deaths per year; 27% 5-year survival), breast (45 Māori deaths per year; 86% 5-year survival), pancreatic (44 Māori deaths per year; 9% 5-year survival), colon (43 Māori deaths per year; 53% 5-year survival), and liver (41 Māori deaths per year; 20% 5-year survival).
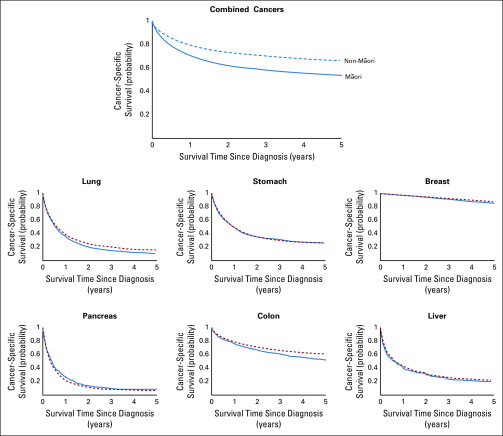
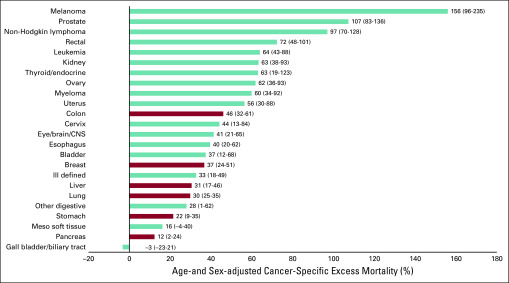
Kaplan-Meier curves of 5-year cancer-specific survival by ethnicity are shown in Figure 1 both for combined cancers and for the 6 cancers with the greatest mortality burden for Māori. When adjusted for age and sex, we observed that Māori were more than twice as likely to die after a cancer diagnosis as non-Māori (adjusted HR, 2.04; 95% CI, 1.99 to 2.09), with excess mortality ranging from 12% to 156% across cancers (Fig 2).
FIG 1.
Crude Kaplan-Meier curves that compare 5-year survival between Māori and non-Māori patients with cancer for all cancers combined as well as for the top 6 highest mortality cancers for Māori.
FIG 2.
Forest plot of age- and sex-adjusted excess cancer-specific mortality (with 95% CIs) experienced by Māori compared with non-Māori by cancer type. The top 6 highest mortality cancers for Māori are shown with red bars.
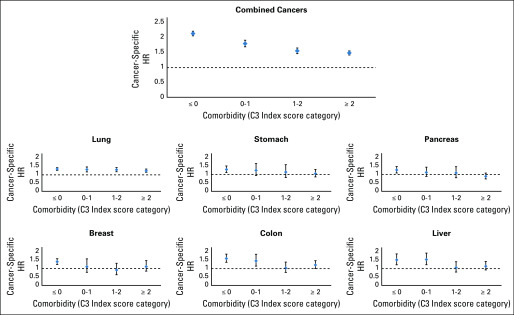
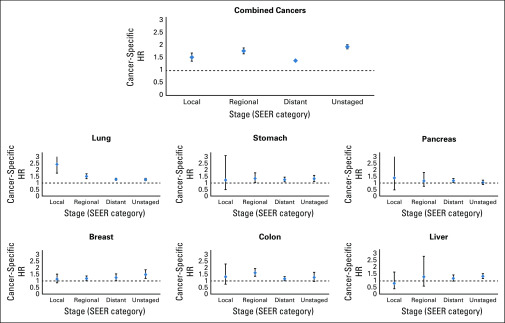
When cancers are combined together, Māori had substantially poorer survival than non-Māori across all levels of area deprivation (Fig 3). However, there was no discernible gradient of increasing (or decreasing) survival disparities with increasing deprivation. In contrast, a gradient can be observed with increasing level of patient comorbidity (Fig 4). When cancers are combined, Maori have poorer survival than non-Māori regardless of stage of disease at diagnosis (Fig 5). However, this pattern was more variable when examining individual cancers.
FIG 3.
Age- and sex-adjusted cancer-specific mortality hazard ratios (HRs; Māori v non-Māori) by deprivation decile for all cancers combined as well as the top 6 highest mortality cancers for Māori. Note that some CI bars are abridged because of standardization of y-axis length between plots. NZDep, New Zealand Deprivation Index.
FIG 4.
Age- and sex-adjusted cancer-specific mortality hazard ratios (HRs; Māori v non-Māori) by comorbidity burden (Cancer and Chronic Conditions [C3] Index score category) for all combined cancers as well as the top 6 highest mortality cancers for Māori.
FIG 5.
Age- and sex-adjusted cancer-specific mortality hazard ratios (HRs; Māori v non-Māori) by stage of disease for all combined cancers as well as the top 6 highest mortality cancers for Māori. Note that some CI bars are abridged because of standardization of y-axis length between plots. Because stage of disease at diagnosis is categorized as not applicable for blood cancers (eg, non-Hodgkin lymphoma), these cancers were excluded from the combined cancers component of this figure.
Cancer-Specific Disparities
Lung cancer.
Māori patients with lung cancer were 30% more likely to die than non-Māori patients with lung cancer. Although there was no discernible survival disparity between Māori patients living in the least-deprived areas, Māori in all other areas were 22%-32% more likely to die than non-Māori. Māori with lung cancer were more likely to die (than non-Maori) across all levels of comorbidity. Survival disparities were present across all stages of disease at diagnosis.
Stomach cancer.
Māori patients with stomach cancer were 22% more likely to die than non-Māori patients with stomach cancer. When stratified by deprivation quintile, the strongest disparity was observed for those in the least-deprived areas (71%), although the small number of patients in this group means that these results have a wide CI (10% to 170%). In terms of comorbidity, the strongest disparities were observed for Māori with little or some comorbidity (C3 Index score < 1). Māori patients seem to have poorer survival across all stages of disease at diagnosis.
Breast cancer.
Māori women with breast cancer were 37% more likely to die than non-Māori women with breast cancer. This disparity is more or less consistent across levels of deprivation. Although disparities among those with some level of comorbidity are either less pronounced or nonexistent, Māori women with no comorbidity are 41% more likely to die than non-Māori women without comorbidity. Consistent disparities in the range of 15%-25% were observed across stages of disease, with the strongest disparity occurring among unstaged patients (50%).
Pancreatic cancer.
Māori patients were 12% more likely to die after a diagnosis of pancreatic cancer than non-Māori patients. Compared with other cancers, the magnitude of this disparity is not large; however, when stratifying by comorbidity, we found that Māori patients with no comorbidity were approximately 30% more likely to die than non-Māori patients with no comorbidity. With respect to stage, further examination of the data reveals that among those with a C3 Index score of 0, there was little or no difference between Māori and non-Māori (eg, localized disease: Māori 3%, non-Māori 2%; distant disease: Māori 61%, non-Māori 56%).
Colon cancer.
Māori patients with colon cancer were 46% more likely to die than non-Māori patients with colon cancer. While no disparities were evident among Māori and non-Māori living in the least-deprived areas, strong disparities persisted for all other deprivation levels. Survival disparities were strongest among those with little or no comorbidity (C3 Index score < 1); further examination reveals that Māori with a C3 score of < 1 were substantially more likely to be diagnosed with advanced disease than non-Māori with the same score (33% v 22%). There was also evidence that Māori had poorer survival outcomes than non-Māori, regardless of stage of disease, which raises the possibility of unequal access to definitive treatment across stages.
Liver cancer.
Māori patients were 31% more likely to die after a diagnosis of liver cancer than non-Māori patients. This disparity was irregular across deprivation quintiles but strongest among those with little or no comorbidity (C3 Index score < 1), where Māori were 53%-55% more likely to die than non-Māori. Further examination reveals that among these patients with little or no comorbidity, Māori seem marginally less likely to be diagnosed with localized disease (2% v 6%) and more likely to be diagnosed with distant disease (37% v 31%) than non-Māori.
DISCUSSION
On the basis of the latest available evidence (2007-2016), Māori continue to have poorer survival than non-Māori for nearly all the most common causes of Māori cancer death (23 of 24 cancers), with the extent of this disparity ranging from 12% to 156% when adjusted for age and sex. These observations are in keeping with those of Robson et al10 for the period 1996-2007, which suggests that these survival disparities persist despite heightened attention over the previous decade. The key drivers of these disparities occur across the cancer continuum and farther upstream, including institutionalized racism in the patterning of the social determinants of health and access to and through high-quality cancer care for Māori.22
We have described how disparities in survival are patterned by (and within) 3 proximal drivers of cancer outcomes (deprivation, comorbidity, and stage of disease). While Māori have poorer cancer survival across all levels of area deprivation when cancers are grouped together, appraisal of the top 6 highest mortality cancers for Māori suggests that this observation is not transferable to all cancers. Deprivation is still an important contributor to poor survival outcomes23 because Māori are more likely to live in deprivation, but the lack of a strong deprivation gradient suggests that the impact of poverty on factors such as early detection and treatment may not meaningfully differ between Māori and non-Māori.
The magnitude of survival disparities between Māori and non-Māori varied across comorbidity categories. Māori without comorbidity tended to be more likely to die as a result of their cancer compared with non-Māori without comorbidity, with the most likely explanation being that non-Māori without comorbidity have better access to early detection of their cancers, and to best practice treatment beyond diagnosis, through factors such as socioeconomic affluence, geographic location, and other conduits.24 There was a tendency for Māori to have poorer survival than non-Māori across all stages of disease, with no consistent pattern with regard to higher disparity for one stage of disease over another.
Deprivation, comorbidity, and stage of disease are strongly intertwined. For example, it is likely that a partial reason why Māori patients with localized lung cancer have poorer survival outcomes than non-Māori patients with the same stage is that these Māori patients are more likely to have comorbidity (and, therefore, may be less likely to be offered treatment, even where it is indicated9), and they are more likely to experience high deprivation (and, therefore, face greater barriers to accessing best practice care). However, the relationship between these key factors (and others) will differ depending on the cancer context.
For brevity, we have outlined the key observations for each investigated cancer in the Data Supplement as well as some specific recommended actions that are needed to help to close the survival gap for Māori. We have summarized these key observations and actions as follows:
Lung cancer: Strong survival disparities at each stage of disease suggest unequal access to curative treatment. A key action to address this includes the finalization and implementation of updated standards of care and care quality indicator monitoring for lung cancer, monitored and reported by ethnicity.
Stomach cancer: Like lung cancer, survival disparities occur at each stage of disease, which again suggests unequal access to treatment. Current provisional standards were published in 2013 with no clear plan for ongoing monitoring and reporting by ethnicity25; this must be addressed as a matter of urgency. These standards cover other upper-GI cancers (including liver cancer), which heightens this urgency.
Breast cancer: Māori patients with breast cancer with no comorbidity were substantially more likely to die than non-Māori patients with no comorbidity, and access to early detection (including screening26) is likely to at least partially explain this disparity.14 As such, achievement of equity in access to early detection (including the BreastScreen Aotearoa program27) is a key flashpoint for addressing survival disparities for this cancer.
Pancreatic cancer: Similar observations with regard to those with no comorbidity were made for pancreatic cancer; however, there is no difference in stage distribution between groups, which suggests that uneven access to early diagnosis is not the driver of the observed survival disparities. Unequal access to treatment is the more likely culprit (backed by evidence of ethnic inequities in other countries28), but this requires further investigation. The creation of new evidence here could inform pancreatic cancer–specific care quality indicators for ongoing monitoring (including by ethnicity).
Colon cancer: Māori have poorer survival outcomes than non-Māori regardless of stage of disease, which raises the possibility of unequal access to definitive treatment across stages (and reinforces the need for equity-focused monitoring of the newly published bowel cancer standards of care and quality performance indicators29).
Liver cancer: The strongest survival disparity occurred among those with little or no comorbidity, and Māori without comorbidity were more likely to be diagnosed with advanced disease than non-Māori. This suggests that early detection may explain some of the excess mortality experienced by Māori. One avenue by which this may occur is through inadequate surveillance of hepatitis among Māori: We previously observed that little more than one third (37%) of Māori patients with hepatitis B–positive liver cancer were on surveillance for their hepatitis before their cancer diagnosis.12 This highlights an urgent unmet need that could improve access to early detection of liver cancer for Māori (and lead to associated improvements in survival outcomes).
Our study used high-quality national-level cancer registry and mortality data to describe population-level disparities in cancer survival between Māori and non-Māori New Zealanders. Potential sources of bias, including length time, lead time, and the selection of cause-specific survival analyses, are further discussed in the Data Supplement for brevity. Finally, we note that our measure of deprivation is based on the area in which people live. While this is likely to reflect the sociodemographic environment in which individuals are living, it is not synonymous with individual-level deprivation, and as such, we may have misclassified some individuals as deprived when they were not (and vice-versa).
In conclusion, Māori patients experience poorer survival outcomes for 23 of the top 24 highest mortality cancers for Māori, with excess mortality across these cancers ranging from 12% to 156%. The magnitude of these survival disparities vary according to deprivation, comorbidity, and stage of disease as well as the cancer context. Of note, there was a tendency for survival disparities to be largest among those with no comorbidity. Although access to early detection and best practice treatment is a common theme, a cancer-specific approach is required to fully understand (and remediate) the survival disparities experienced by Māori for a given cancer. As such, we have provided key areas of required action for each of the most common causes of cancer death for Māori.
ACKNOWLEDGMENT
We acknowledge Chris Lewis of the Ministry of Health’s National Collections team for assistance with data extraction.
SUPPORT
Supported by the Health Research Council of New Zealand (HRC reference #18/588).
AUTHOR CONTRIBUTIONS
Conception and design: Jason Gurney, James Stanley, Jonathan Koea, Chris Jackson, Diana Sarfati
Collection and assembly of data: Jason Gurney
Data analysis and interpretation: Jason Gurney, Melissa McLeod, Jonathan Koea, Chris Jackson, Diana Sarfati
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
James Stanley
Stock and Other Ownership Interests: Gains Psychology Lower Hutt, New Zealand (I)
Melissa McLeod
Stock and Other Ownership Interests: City GPs Limited (I)
Chris Jackson
Honoraria: MSD Oncology, Athenex
Research Funding: Athenex (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending Oraxol, Athenex, no financial interest
Travel, Accommodations, Expenses: Athenex
No other potential conflicts of interest were reported.
REFERENCES
- 1.Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019;20:1493–1505. doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyratzopoulos G, Neal RD, Barbiere JM, et al. Variation in number of general practitioner consultations before hospital referral for cancer: Findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 2012;13:353–365. doi: 10.1016/S1470-2045(12)70041-4. [DOI] [PubMed] [Google Scholar]
- 3.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: A review. Ann Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 4.Lejeune C, Sassi F, Ellis L, et al. Socio-economic disparities in access to treatment and their impact on colorectal cancer survival. Int J Epidemiol. 2010;39:710–717. doi: 10.1093/ije/dyq048. [DOI] [PubMed] [Google Scholar]
- 5. Vaccarella S, Lortet-Tieulent J, Saracci R, et al (eds): Reducing Social Inequalities in Cancer: Evidence and Priorities for Research. Lyon, France, International Agency for Research on Cancer, 2019. [PubMed] [Google Scholar]
- 6.Ellis L, Canchola AJ, Spiegel D, et al. Racial and ethnic disparities in cancer survival: The contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36:25–33. doi: 10.1200/JCO.2017.74.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkins GT, Kim T, Munson J. Residence in rural areas of the United States and lung cancer mortality: Disease incidence, treatment disparities, and stage-specific survival. Ann Am Thorac Soc. 2017;14:403–411. doi: 10.1513/AnnalsATS.201606-469OC. [DOI] [PubMed] [Google Scholar]
- 8.Turner M, Fielding S, Ong Y, et al. A cancer geography paradox? Poorer cancer outcomes with longer travelling times to healthcare facilities despite prompter diagnosis and treatment: A data-linkage study. Br J Cancer. 2017;117:439–449. doi: 10.1038/bjc.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66:337–350. doi: 10.3322/caac.21342. [DOI] [PubMed] [Google Scholar]
- 10.Robson B, Purdie G, Cormack D. Unequal Impact II: Māori and Non-Māori Cancer Statistics by Deprivation and Rural-Urban Status, 2002-2006. Wellington, New Zealand: Ministry of Health; 2010. [Google Scholar]
- 11.Hill S, Sarfati D, Blakely T, et al. Survival disparities in Indigenous and non-Indigenous New Zealanders with colon cancer: The role of patient comorbidity, treatment and health service factors. J Epidemiol Community Health. 2010;64:117–123. doi: 10.1136/jech.2008.083816. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain J, Sarfati D, Cunningham R, et al. Incidence and management of hepatocellular carcinoma among Māori and non-Māori New Zealanders. Aust N Z J Public Health. 2013;37:520–526. doi: 10.1111/1753-6405.12108. [DOI] [PubMed] [Google Scholar]
- 13.Signal V, Sarfati D, Cunningham R, et al. Indigenous inequities in the presentation and management of stomach cancer in New Zealand: A country with universal health care coverage. Gastric Cancer. 2015;18:571–579. doi: 10.1007/s10120-014-0410-y. [DOI] [PubMed] [Google Scholar]
- 14.Tin Tin S, Elwood JM, Brown C, et al. Ethnic disparities in breast cancer survival in New Zealand: Which factors contribute? BMC Cancer. 2018;18:58. doi: 10.1186/s12885-017-3797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarfati D, Gurney J, Stanley J, et al. A retrospective cohort study of patients with stomach and liver cancers: The impact of comorbidity and ethnicity on cancer care and outcomes. BMC Cancer. 2014;14:821. doi: 10.1186/1471-2407-14-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ministry of Health: New Zealand Cancer Registry (NZCR), 2020 https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/new-zealand-cancer-registry-nzcr.
- 17.Ministry of Health . Mortality Collection Data Dictionary, v1.7. Wellington, New Zealand: Ministry of Health; 2019. [Google Scholar]
- 18.Salmond CE, Crampton P. Development of New Zealand’s deprivation index (NZDep) and its uptake as a national policy tool. Can J Public Health. 2012;103:S7–S11. [PubMed] [Google Scholar]
- 19.Sarfati D, Gurney J, Stanley J, et al. Cancer-specific administrative data-based comorbidity indices provided valid alternative to Charlson and NHI indices. J Clin Epidemiol. 2014;67:586–595. doi: 10.1016/j.jclinepi.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 20. Young J, Roffers F, Gloeckler Ries L, et al (eds): SEER Summary Staging Manual – 2000: Codes and Coding Instructions. Bethesda, MD, National Cancer Institute, 2000. [Google Scholar]
- 21.Gurney J, Sarfati D, Stanley J. The impact of patient comorbidity on cancer stage at diagnosis. Br J Cancer. 2015;113:1375–1380. doi: 10.1038/bjc.2015.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurney J, Campbell S, Jackson C, et al. Equity by 2030: Achieving equity in survival for Māori cancer patients. N Z Med J. 2019;132:66–76. [PubMed] [Google Scholar]
- 23.Jeffreys M, Sarfati D, Stevanovic V, et al. Socioeconomic inequalities in cancer survival in New Zealand: The role of extent of disease at diagnosis. Cancer Epidemiol Biomarkers Prev. 2009;18:915–921. doi: 10.1158/1055-9965.EPI-08-0685. [DOI] [PubMed] [Google Scholar]
- 24. Gurney J, Campbell S, Jackson C, et al: Equity by 2030: Achieving equity in survival for Māori cancer patients. N Z Med J 135:66-76, 2019. [PubMed] [Google Scholar]
- 25.National HBP/Upper GI Tumour Standards Working Group . Standards of Service Provision for Upper Gastrointestinal Cancer Patients in New Zealand - Provisional. Wellington, New Zealand: Ministry of Health; 2013. [Google Scholar]
- 26.Seneviratne S, Campbell I, Scott N, et al. Impact of mammographic screening on ethnic and socioeconomic inequities in breast cancer stage at diagnosis and survival in New Zealand: A cohort study. BMC Public Health. 2015;15:46. doi: 10.1186/s12889-015-1383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robson B, Stanley J: BreastScreen Aotearoa Programme Monitoring Report: For Maori, Pacific and Total women screened during the two or four years to June 2016. Te Rōpū Rangahau Hauora a Eru Pōmare. Wellington, New Zealand, University of Otago, 2017. [Google Scholar]
- 28.Murphy MM, Simons JP, Hill JS, et al. Pancreatic resection: A key component to reducing racial disparities in pancreatic adenocarcinoma. Cancer. 2009;115:3979–3990. doi: 10.1002/cncr.24433. [DOI] [PubMed] [Google Scholar]
- 29. Ministry of Health: Cancer Quality Performance Indicator Programme Wellington, 2019 https://www.health.govt.nz/our-work/diseases-and-conditions/national-cancer-programme/cancer-initiatives/cancer-quality-performance-indicator-programme.