Fig. 1.
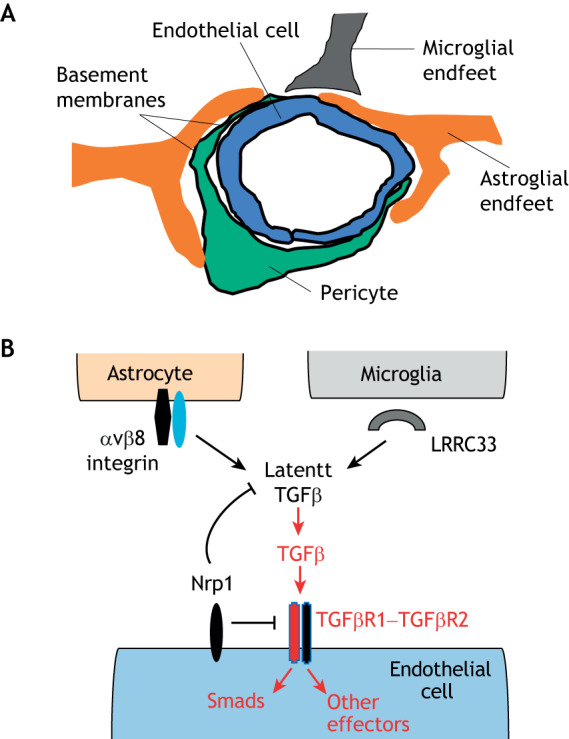
Integrin αvβ8 regulates neurovascular development via activation of latent TGFβs. (A) Schematic illustration of the multicellular composition of a brain neurovascular unit, comprised of vascular endothelial cells and pericytes that are juxtaposed with astrocyte and microglial end feet. There are at least two basement membranes (highlighted by dark black lines) within the neurovascular unit, highlighting the importance of cell–ECM contact and communication in neurovascular biology. (B) αvβ8 integrin expressed in perivascular astroglial cells cooperates with microglial cell-expressed and secreted LRRC33 to bind to latent TGFβs embedded within the ECM. Interactions between integrin and LRRC33 induce structural rearrangements within the latent TGFβ complex, leading to paracrine activation of TGFβ receptor signaling in vascular endothelial cells. TGFβ receptor signaling via Smad transcription factors and other effectors leads to changes in gene expression programs that regulate angiogenesis during CNS development. Nrp1, which is expressed in brain endothelial cells, suppresses TGFβ signaling by serving as a counter-receptor for αvβ8 integrin, thereby blocking latent TGFβ activation. Genetic or pharmacological disruption of integrin engagement of TGFβ receptor signaling leads to severe CNS angiogenesis pathologies. The TGFβ receptor complex consists of a heterodimer of type 1 TGFβ receptor (indicated in red) and type 2 TGFβ receptor (indicated in black).
