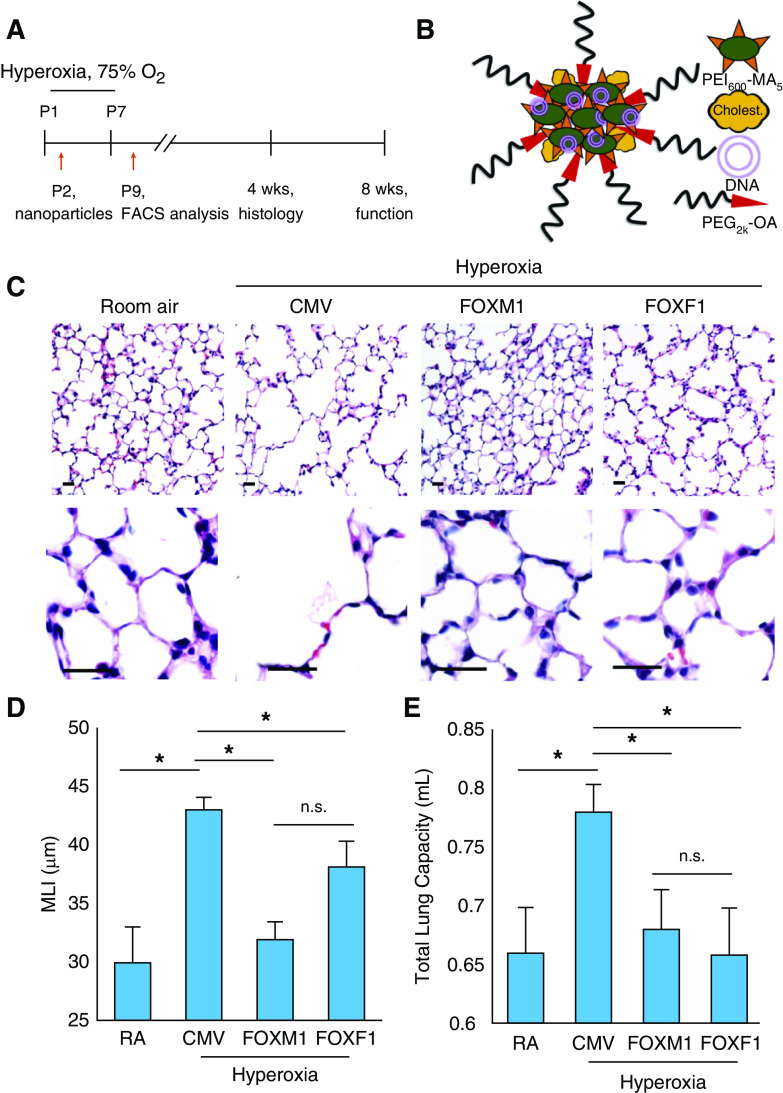
Figure 1.
Nanoparticle delivery of FOXM1 (forkhead box M1) or FOXF1 (forkhead box F1) decreases alveolar simplification caused by neonatal hyperoxia. (A) Schematic representation of 7-day hyperoxia treatment of wild-type newborn mice. Control mice were exposed to room air (RA). Polyethylenimine-(5) myristic acid/polyethylene glycol oleic acid/cholesterol (PEI600-MA5/PEG-OA/Cho) nanoparticles were delivered at Postnatal Day 2 (P2) via the facial vein. (B) Structure of PEI600-MA5/PEG-OA/Cho nanoparticles containing plasmid DNA, PEI600-MA5, and polyethylene glycol oleic acid (PEG2k-OA). (C) Hematoxylin and eosin (H&E) staining of paraffin-embedded lung sections shows alveolar simplification in hyperoxia-treated mice. Mice were exposed to hyperoxia or RA from P1 to P7, followed by RA exposure until lung harvest at P28. Nanoparticle delivery was performed at P2. Delivery of either FOXM1 or FOXF1 expression vectors improves lung structure in hyperoxia-treated mice compared with cytomegalovirus (CMV)-empty control. Scale bars, 50 μm. (D) Nanoparticle delivery of FOXM1 or FOXF1 decreases alveolar simplification. Mean linear intercept (MLI) was calculated using 15 random H&E–stained lung fields (n = 4–6 mice per group). (E) Nanoparticle delivery of FOXM1 or FOXF1 normalizes TLC in hyperoxia-treated mice. TLC was measured using the flexiVent ventilator when mice were 8 weeks of age (n = 4–6 mice per group). Error bars are mean ± SE. *P < 0.05. FACS = fluorescence-activated cell sorter; n.s. = not significant.