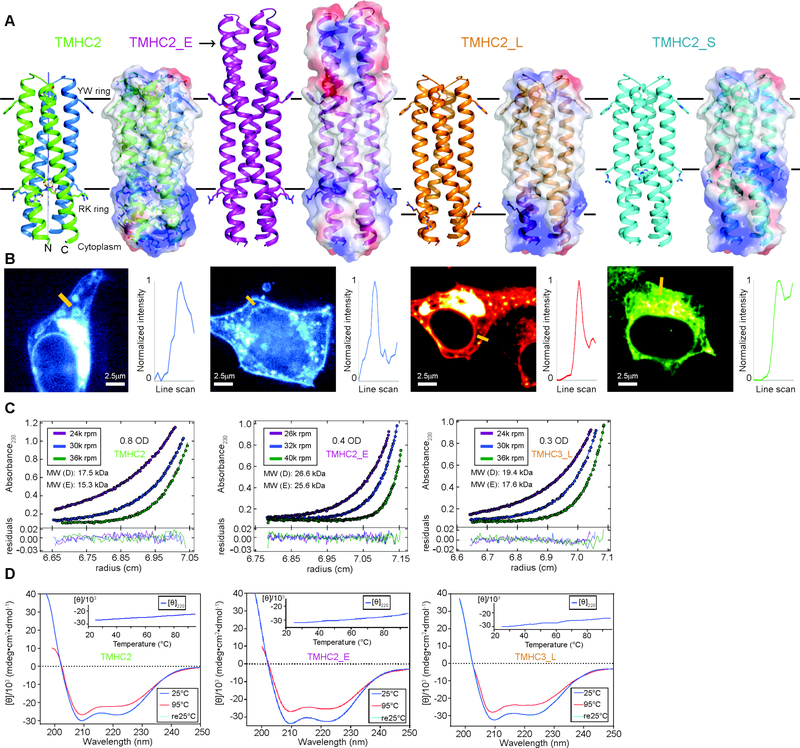
Fig. 1.
Design and characterization of proteins with four transmembrane helices. From left to right, designs and data are shown for TMHC2 (transmembrane hairpin C2), TMHC2_E (elongated), TMHC2_L (long span) and TMHC2_S (short span). (A) Design models with intra- and extra-membrane regions with different lengths. Horizontal lines demarcate the hydrophobic membrane regions. Ribbon diagrams are on left, electrostatic surfaces on right, and the neutral transmembrane regions are in gray. (B) Confocal microscopy images for HEK293T cells transfected with TMHC2 fused to mTagBFP, TMHC2_E fused to mTagBFP, TMHC2_L fused to mCherry and TMHC2_S fused to eGFP. Line scans (yellow lines in the images) across the membranes show significant increase in fluorescence across the plasma membranes for TMHC2, TMHC2_E and TMHC2_L, but less significant increase for TMHC2_S. (C) Representative analytical ultracentrifugation sedimentation-equilibrium curves at three different rotor speeds. Each data set is globally well fitted as a single ideal species in solution corresponding to the dimer molecular weight. ‘MW (D)’ and ‘MW (E)’ indicate the molecular weight of the oligomer design and that determined from experiment, respectively. (D) CD spectra and temperature melt (inset). No apparent unfolding transitions are observed up to 95°C.