Fig. 2.
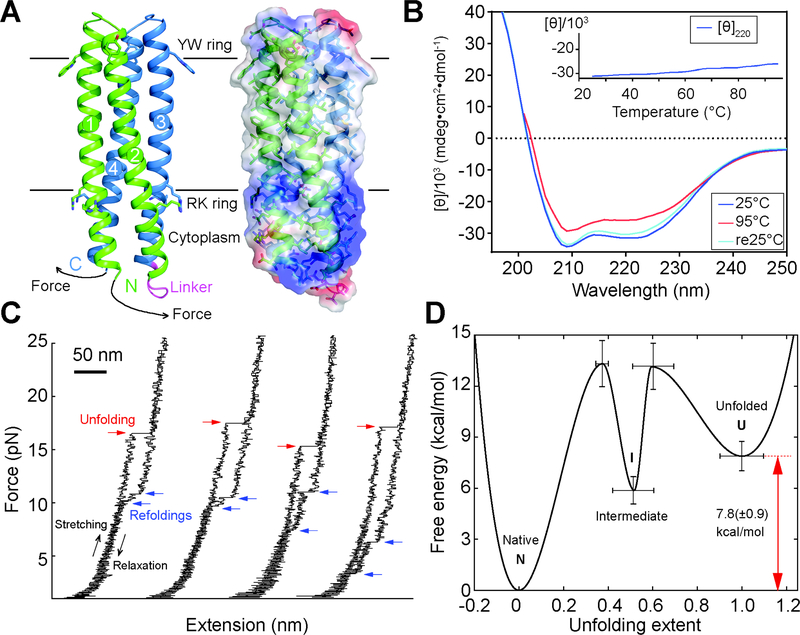
Folding stability of the 156-residue single chain TMHC2 (scTMHC2) design with four transmembrane helices. (A) Design model (left) and electrostatic surface (right) of scTMHC2. N- and C-terminal helical hairpins are colored green and blue respectively. Numbers indicate the order of the four TMs in the sequence. The linker connecting the two hairpins is colored magenta. Single-molecule forced unfolding experiments were conducted by applying mechanical tension to the N- and C-terminus of a single scTMHC2 (Fig. S5 for more details). (B) CD spectra of scTMHC2 at different temperatures. No unfolding transition is observed up to 95°C. (C) Single-molecule force-extension traces of scTMHC2. The unfolding and refolding transitions are denoted with red and blue arrows. (D) Folding energy landscape obtained from the single-molecule experiments. N, I, and U indicate the native, intermediate, and unfolded state respectively.