ABSTRACT
Background
Advances in the treatment of Parkinson's disease (PD) and changes in general life expectancy may have improved survival in patients with PD.
Objective
The objective of this study was to investigate recent trends in PD mortality.
Methods
In total, 1521 patients with PD in local and national registries were followed for 11 years (2006–2016) from diagnosis until exit date or death, and the causes of death were recorded.
Results
The survival of men with PD improved during the follow‐up period, but no change was observed in women (2‐year postdiagnosis survival in men, 79.0%–86.3%, P = 0.03; 2‐year postdiagnosis survival in women, 82.8%–87.5%, P = 0.42). Pneumonia was the most common immediate cause of death.
Discussion
The survival of men with PD has improved in Finland without a similar change in women. Because changes in treatment likely affect both sexes similarly, the results may reflect the decreasing sex gap in life expectancy. This phenomenon will likely increase the already high male‐to‐female prevalence ratio of PD.
Keywords: Parkinson's disease, survival, mortality, gender, sex
Parkinson's disease (PD) is associated with increased mortality, as, on average, survival is reduced by approximately 5% every year in followed cohorts.1 Although studies have demonstrated the effectiveness of monoamine oxidase‐B inhibitors,2, 3, 4, 5, 6 dopamine agonists,7, 8, 9, 10, 11 and levodopa12, 13 in the earlier stages of PD, the effects of antiparkinsonian medications on PD mortality remain speculative. In recent years, in line with new evidence, the traditional view of early pharmacotherapy has started to shift from delayed levodopa at advanced stages of PD to earlier initiation of low‐dose therapy for improved quality of life.14, 15, 16 Otherwise, the primary pharmacotherapy for early and moderate PD has remained mostly unaltered for the past two decades in developed countries,17, 18, 19 and there are no sex‐specific recommendations for drug therapy in PD.20
The greatest improvements in PD treatment have recently been documented in patients with advanced PD. Device‐aided therapies have induced treatment effects that appear to surpass those of optimal oral drug therapy in advanced PD.21, 22, 23, 24, 25 However, although deep brain stimulation and pump therapies induce clear effects on quality of life in advanced PD, there is a similar lack of current evidence for increased survival.21, 23, 24, 25, 26 Nevertheless, there are potentially highly relevant changes in treatment that could affect mortality, namely, improved rehabilitation, fall prevention, and changes in the end‐of‐life care for patients with PD.27, 28, 29, 30, 31
It is hypothesized that changes in the care of PD have increased the life expectancy (LE) of patients with PD. Nevertheless, as a late‐life neurodegenerative disease, PD epidemiology is also affected by general factors that increase or decrease LE. Moreover, the sex gap in LE has been reported to narrow.32 Here, we investigated possible changes in the mortality of a large hospital‐based cohort of Finnish patients with PD in three different time points with respect to time of PD diagnosis by following a total of 1521 patients with PD in local and national registries for 11 years and taking into account the effects of device‐aided therapies, causes of death, and general changes in LE.
Methods
The Turku Clinical Research Center (http://www.turkucrc.fi/en) maintains the Turku University Hospital database, which stores all electronic health records generated at the hospital for medical research. Health records have been in electronic format from January 1, 2004, onward, enabling annual longitudinal analyses, and they include demographics, clinical diagnoses and procedures, inpatient periods and outpatient visits, pathology diagnoses and reports, imaging reports, chemotherapy and radiation treatments, inpatient medications and outpatient prescriptions, laboratory measurements, and clinical narratives. At present, the database contains detailed clinical data of approximately 0.5 million patients who have visited Turku University Hospital or regional hospitals. The data are pseudonymized, protecting the identity of the patients while making it possible to link data elements to individual patients.
The study population consisted of patients with PD diagnosed between 2006 and 2014 at Turku University Hospital in southwestern Finland. The hospital district has a population base of approximately 470,000 people. Our study cohort included only patients from the university hospital database, and the great majority of diagnoses were based on outpatient visits. We employed a methodological approach that involved the investigations of complete, digital, detailed individual health records during a period of 11 years. Patients with PD were identified from the digital database using the International Classification of Diseases 10th Revision code G20. All diagnoses of PD were based on clinical examination by a certified neurologist using the UK Brain Bank Criteria or Movement Disorder Society clinical diagnostic criteria.33 The date of diagnosis was defined as the first appearance of the diagnosis code in the patient records. After identifying patients and diagnosis dates, all individual clinical data were collected from the database. Dates and individual causes of death were obtained from the national authority, Statistics Finland (www.stat.fi). The beginning of the follow‐up period was January 1, 2006, and the end of the follow‐up period was set to December 31, 2016.
To investigate possible longitudinal changes in survival, the cohort was divided into the following three groups based on the year of diagnosis: 2006 to 2008, 2009 to 2011, and 2012 to 2014. Data analysis was separately performed for a sample in which patients with device‐aided therapies were excluded. The overall survival (OS) was defined as the percentage of patients who were still alive after their diagnosis at the following time points: 1, 2, and 4 years. Hence, survival was investigated identically for every patient with PD at 1 and 2 years from the diagnosis. Therefore, our main result of the OS at 2 years after diagnosis has been evaluated similarly and is valid for all patients. The 4‐year time point was evaluated similarly for the 2006 to 2008 and 2009 to 2011 groups. However, because the end of our follow‐up was set at December 31, 2016, the result of the OS 4 years after diagnosis in patients with PD diagnosed between 2012 and 2014 was estimated using the Kaplan‐Meier analysis. To investigate possible differences in the correctness of PD diagnoses between men and women, changed or removed diagnoses were investigated within a 2‐year timeline in 2011 (midpoint of the follow‐up period). We also analyzed potential changes in antiparkinsonian medication according to sex.
The study was approved by Turku Clinical Research Center (TT263/2017, diary number TK‐53‐652‐18). Because the study did not involve patient contacts, approval of the ethics committee was not required.
Continuous and categorical variables were compared between groups using analysis of variance and chi‐square tests, respectively. Kaplan‐Meier analysis was used to analyze changes in the OS. Log‐rank tests were used to calculate the P values between survival curves. The level of statistical significance was set to P < 0.05. All statistical analyses were performed with IBM SPSS Statistics for Windows (version 25.0, 2017, IBM Corp., Armonk, NY).
Results
We identified 1521 patients with PD (54.2% men; Table 1, Fig. 1). The number of new diagnoses of PD per year remained stable during the study period (men, 261–292 diagnoses per three years, P = 0.14; women, 216–256 diagnoses per three years, P = 0.14). Men were diagnosed at a younger age than women (71.6 years [SD, 10.5 years] vs. 74.1 years, [SD, 10.4 years]; P < 0.01), but the age difference and mean age at diagnosis remained stable (men, P = 0.41; women, P = 0.84). In 2011, at the midpoint of the follow‐up period, 55 men and 54 women were diagnosed with PD. Two years later, the diagnoses remained unaltered for 42 of men (76.4%) and 42 of women (77.8%). There were no significant differences in the diagnostic inaccuracy between men and women (men, 23.6%; women, 22.2%; P = 0.86; Supplementary Table S1). During the 11‐year study period, 685 patients died (47.1% of men and 42.6% of women). The OS of male patients with PD increased during the follow‐up period (P = 0.03), but no change was observed in women (P = 0.42; Table 1, Fig. 1). The hazard ratio for men in the last follow‐up period was 0.71 (95% confidence interval, 0.53–0.95; P = 0.02), indicating better survival for men if the diagnosis was made later in the follow‐up period. The results remained the same when patients receiving device‐aided therapies (n = 29) were excluded from the analysis. The number of patients with PD using levodopa and dopamine agonists remained stable during the study period in both sexes (levodopa: men, 55.2%–60.6% per three years, P = 0.39 and women, 54.2%–60.2% per three years, P = 0.41; dopamine agonists: men, 40.6%–43.8% per three years, P = 0.72 and women, 38.2%–41.8% per three years, P = 0.24; Supplementary Table S2).
Table 1.
Demographic characteristics of the patients and changes in the overall survival according to the year of PD diagnosis
| Year of Diagnosis | Diagnoses, M/W, n (%) | Age at Diagnosis, M/W, (y) | Deaths, M/W, n | Overall Survival, M/W, %a | Hazard Ratio (95% CI, P), M/W | ||
|---|---|---|---|---|---|---|---|
| 1 Year After Diagnosis | 2 Years After Diagnosis | 4 Years After Diagnosis | |||||
| 2006–2008 | 271/256 (51.4/48.6) | 72.2/74.3 | 191/154 | 86.0/89.8 | 79.0/82.8 | 62.0/69.1 | – |
| 2009–2011 | 261/225 (53.7/46.3) | 71.7/74.2 | 122/96 | 90.4/94.2 | 82.8/88.4 | 67.8/74.7 | 0.80 (0.63–1.01, 0.057)/0.92 (0.70–1.20, 0.534) |
| 2012–2014 | 292/216 (57.5/42.5) | 71.0/73.8 | 75/47 | 91.1/93.1 | 86.3/87.5 | 71.5/77.3 | 0.71 (0.53‐0.95, 0.019)/0.79 (0.56–1.12, 0.191) |
| Total | 824/697 (54.2/45.8) | 71.6/74.1 | 388/297 | – | |||
P value for Kaplan‐Meier analysis: M, P = 0.03; W, P = 0.42.
M, men; W, women; Cl, confidence interval.
Figure 1.
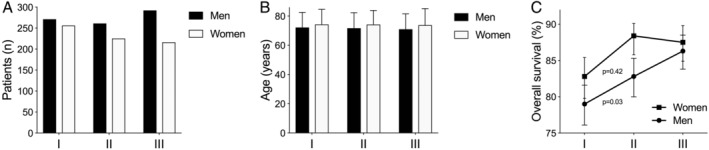
Demographic characteristics of the sample according to the year of Parkinson's disease diagnosis: (A) number of new diagnosis, (B) age at diagnosis, and (C) overall survival 2 years after diagnosis according to follow‐up period. Note the increased overall survival in men but not in women in C. I = diagnosis years 2006 to 2008, II = diagnosis years 2009 to 2011, III = diagnosis years 2012 to 2014.
Pneumonia was the most common immediate cause of death in all 3 time periods in both men and women (38.2% of deaths in men and 25.9% of deaths in women; Table 2). The second and third most common causes of death were heart failure and myocardial infarction, respectively. There were no marked changes in the causes of death during the study period. PD was reported as a contributing factor to death in 36.3% of men and 38.4% of women, and the percentage did not change significantly during the follow‐up period (men, 40.3% vs. 33.6% vs. 30.7%, P = 0.25; women, 41.6% vs. 35.4% vs. 34.0%, P = 0.50; Table 2).
Table 2.
The 3 most common immediate causes of death in the studied cohorts (I, II, III) in patients with PD according to sex and year of diagnosis
| Cohort | PD as a Contributing Factor, n/% | ||||
|---|---|---|---|---|---|
| Year of Diagnosis | Gender | I, n/% | II, n/% | III, n/% | |
| 2006–2008 | M | Pneumonia (72/37.7) | HF (7/3.7) | MI (3/1.6) | 77/40.3 |
| W | Pneumonia (45/29.2) | HF (5/3.2) | MI (5/3.2) | 64/41.6 | |
| 2009–2011 | M | Pneumonia (49/40.2) | MI (5/4.1) | CA (2/1.6) | 41/33.6 |
| W | Pneumonia (22/22.9) | MI (3/3.1) | HF (2/2.1) | 34/35.4 | |
| 2012–2014 | M | Pneumonia (27/36.0) | HF (4/5.3) | MI (2/2.7) | 23/30.7 |
| W | Pneumonia (10/21.3) | MI (3/6.4) | HF (2/4.3) | 16/34.0 | |
| Total | M | Pneumonia (148/38.2) | HF (12/3.1) | MI (10/2.6) | 141/36.3 |
| W | Pneumonia (77/25.9) | MI (11/3.7) | HF (9/3.0) | 114/38.4 | |
The immediate cause of death was not reported for 43.6% of men and 57.2% of women.
PD, Parkinson's disease; M, men; W, women; HF, heart failure; MI, myocardial infarction; CA, cardiac arrest.
Discussion
The results demonstrate that the mortality of male Finnish patients with PD has decreased during a period of 11 years. No significant changes in the mortality of female patients with PD were observed. In Finland, the diagnostic criteria and treatment lines of PD are regionally homogeneous and are based on national current care guidelines. Therefore, the result could reflect greater improvements in male survival compared with female survival at the population level, and the same sex differences can likely be seen in other regions with aging PD populations and comparable health care systems.
Competing causes of death refers to an epidemiological fact that if a patient is cured and will not die from one disease, he or she is eligible to have another fatal disease. The most important reason for the demonstrated change in the LE of male patients with PD could be caused by population‐level changes in morbidity and mortality. Between 2006 and 2016, the general LE of Finnish newborns increased by 1.3 years in girls and 2.6 years in boys.34 Similarly, in 73‐year‐old individuals in the general population (mean age of the present PD population), the LE increased almost twice as much in men compared with women (1.1 vs. 0.6 years).32 The present results may thus at least partially be related to the decreasing sex differences in survival. There are several reasons for the increased LE in men in Finland, but successful actions taken for the prevention and control of noncommunicable diseases are probably contributing. Cardiovascular diseases, cancers, chronic respiratory diseases, and diabetes are the most important causes of death worldwide and have affected more men than women.35 In Finland, the most important cause of death has long been ischemic heart disease, and age‐standardized mortality for circulatory diseases has declined, especially in men.36
Studies have reported that there are no differences in the type of dopaminergic medication prescribed to women and men, but men are often medicated with higher doses of levodopa both orally and via infusion.20 This is most likely attributed to the higher average body mass of men.20 However, the differences may not be solely explained by differences in body weight, as there are sex differences in levodopa bioavailability, irrespective of body weight.37, 38 In addition, men are more often treated with deep brain stimulation than women in PD, although women more commonly suffer from motor fluctuations and dyskinesia.20, 39 Diagnostic conventions and pharmacotherapy for PD remained essentially the same in Finland during the study period, as did the proportion of male and female patients with PD using levodopa and dopamine agonists in our cohort. We do not suspect that sex differences in therapy were a major contributing factor in the results. Moreover, the results remained the same when the small number of patients treated with device‐aided therapies were excluded from the analysis. We did observe a slight difference in the ages at diagnosis between men and women (mean difference of 2.5 years; men were diagnosed earlier). It has been suggested that the phenotype of PD in women may be more benign and that symptoms may begin later for women than in men, which could explain this difference.20 It should be noted that nonpharmacological therapies, including physiotherapy, speech therapy, and occupational therapy, are given mainly in local health care centers in Finland. These data were not available for us, and this can be considered as a limitation of our study because the convention and intensity of these therapies might have changed during the follow‐up period.
In summary, the present study shows that there is disparity in mortality between male and female patients with PD, which will probably lead to an increasing male‐to‐female ratio in PD prevalence.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
T.K.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
S.K.: 1C, 2A, 2B, 2C, 3B
J.S.: 1C, 2C, 3B
H.S‐M.: 1B, 1C, 2C, 3B
O.C.: 1B, 1C, 2C, 3B
V.K.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
Disclosures
Ethical Compliance Statement
The authors confirm that the approval of an institutional review board was not required for this work. The study was approved by Turku Clinical Research Center (TT263/2017, diary number TK‐53‐652‐18). Informed consent was not required. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest
This study was funded by The Finnish Parkinson Foundation and Turku University Hospital (TYKS Foundation). The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for Previous 12 Months
T.K., S.K., and H.S‐M. declare that there are no disclosures to report. J.S. has stock ownership in Orion Corporation. O.C. has stock ownership in Orion Corporation and has received honoraria from Roche Pharma. V.K. has been part of advisory board of Abbvie and has received consulting fees from Lundbeck, travel expenses from Nordic Infucare AB, and speaker's honoraria from Abbvie and Nordic Infucare AB.
Supporting information
Supplementary Table S1. The percentage of patients with unchanged PD diagnoses over a 2‐year follow‐up period (2011–2013) according to sex.
Supplementary Table S2. The number and relative proportion of patients with PD using levodopa and dopamine agonists according to sex and year of diagnosis.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson's disease: a systematic review and meta‐analysis. Mov Disord 2014;29(13):1615–1622. [DOI] [PubMed] [Google Scholar]
- 2. Group PS . Effect of deprenyl on the progression of disability in early Parkinson's disease. N Engl J Med 1989;321(20):1364–1371. [DOI] [PubMed] [Google Scholar]
- 3. Allain H, Pollak P, Neukirch HC. Symptomatic effect of selegiline in de novo parkinsonian patients. The French Selegiline Multicenter Trial. Mov Disord 1993;8(suppl 1):S36–S40. [DOI] [PubMed] [Google Scholar]
- 4. Pålhagen S, Heinonen EH, Hägglund J, et al. Selegiline delays the onset of disability in de novo parkinsonian patients. Swedish Parkinson Study Group. Neurology 1998;51(2):520–525. [DOI] [PubMed] [Google Scholar]
- 5. Parkinson Study Group . A controlled trial of rasagiline in early Parkinson disease: the TEMPO study. Arch Neurol 2002;59(12):1937–1943. [DOI] [PubMed] [Google Scholar]
- 6. Olanow CW, Rascol O, Hauser R, et al. A double‐blind, delayed‐start trial of rasagiline in Parkinson's disease. N Engl J Med 2009;361(13):1268–1278. [DOI] [PubMed] [Google Scholar]
- 7. Parkinson Study Group . Safety and efficacy of pramipexole in early Parkinson disease. A randomized dose‐ranging study. JAMA 1997;278(2):125–130. [DOI] [PubMed] [Google Scholar]
- 8. Shannon KM, Bennett JP, Friedman JH. Efficacy of pramipexole, a novel dopamine agonist, as monotherapy in mild to moderate Parkinson's disease. The Pramipexole Study Group. Neurology 1997;49(3):724–728. [DOI] [PubMed] [Google Scholar]
- 9. Adler CH, Sethi KD, Hauser RA, et al. Ropinirole for the treatment of early Parkinson's disease. The Ropinirole Study Group. Neurology 1997;49(2):393–399. [DOI] [PubMed] [Google Scholar]
- 10. Parkinson Study Group . A controlled trial of rotigotine monotherapy in early Parkinson's disease. Arch Neurol 2003;60(12):1721–1728. [DOI] [PubMed] [Google Scholar]
- 11. Watts RL, Jankovic J, Waters C, Rajput A, Boroojerdi B, Rao J. Randomized, blind, controlled trial of transdermal rotigotine in early Parkinson disease. Neurology 2007;68(4):272–276. [DOI] [PubMed] [Google Scholar]
- 12. Hauser RA, Auinger P, Oakes D, Parkinson Study Group . Levodopa response in early Parkinson's disease. Mov Disord 2009;24(16):2328–2336. [DOI] [PubMed] [Google Scholar]
- 13. Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med 2004;351(24):2498–2508. [DOI] [PubMed] [Google Scholar]
- 14. Schapira AH, Olanow CW. Drug selection and timing of initiation of treatment in early Parkinson's disease. Ann Neurol 2008;64(suppl 2):S47–S55. [DOI] [PubMed] [Google Scholar]
- 15. Cilia R, Akpalu A, Sarfo FS, et al. The modern pre‐levodopa era of Parkinson's disease: insights into motor complications from sub‐Saharan Africa. Brain 2014;137(Pt 10):2731–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gray R, Ives N, Rick C, et al. Long‐term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson's disease (PD MED): a large, open‐label, pragmatic randomised trial. Lancet 2014;384(9949):1196–1205. [DOI] [PubMed] [Google Scholar]
- 17. Rascol O, Lozano A, Stern M, Poewe W. Milestones in Parkinson's disease therapeutics. Mov Disord 2011;26(6):1072–1082. [DOI] [PubMed] [Google Scholar]
- 18. Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386(9996):896–912. [DOI] [PubMed] [Google Scholar]
- 19. Pahwa R, Lyons KE. Treatment of early Parkinson's disease. Curr Opin Neurol 2014;27(4):442–449. [DOI] [PubMed] [Google Scholar]
- 20. Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT. The relevance of gender in Parkinson's disease: a review. J Neurol 2017;264(8):1583–1607. [DOI] [PubMed] [Google Scholar]
- 21. Olanow CW, Kieburtz K, Odin P, et al. Continuous intrajejunal infusion of levodopa‐carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double‐blind, double‐dummy study. Lancet Neurol 2014;13(2):141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antonini A, Moro E, Godeiro C, Reichmann H. Medical and surgical management of advanced Parkinson disease. Mov Disord 2018;33(6):900–908. [DOI] [PubMed] [Google Scholar]
- 23. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009;301(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med 2013;368(7):610–622. [DOI] [PubMed] [Google Scholar]
- 25. Antonini A, Nitu B. Apomorphine and levodopa infusion for motor fluctuations and dyskinesia in advanced Parkinson disease. J Neural Transm (Vienna) 2018;125(8):1131–1135. [DOI] [PubMed] [Google Scholar]
- 26. Obeso JA, Olanow CW, Rodriguez‐Oroz MC, et al. Deep‐brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med 2001;345(13):956–963. [DOI] [PubMed] [Google Scholar]
- 27. Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson's disease: Current outlook and future challenges. Parkinsonism Relat Disord 2016;22(suppl 1):S60–S64. [DOI] [PubMed] [Google Scholar]
- 28. Fasano A, Canning CG, Hausdorff JM, Lord S, Rochester L. Falls in Parkinson's disease: a complex and evolving picture. Mov Disord 2017;32(11):1524–1536. [DOI] [PubMed] [Google Scholar]
- 29. Monticone M, Ambrosini E, Laurini A, Rocca B, Foti C. In‐patient multidisciplinary rehabilitation for Parkinson's disease: a randomized controlled trial. Mov Disord 2015;30(8):1050–1058. [DOI] [PubMed] [Google Scholar]
- 30. Veronese S, Gallo G, Valle A, et al. Specialist palliative care improves the quality of life in advanced neurodegenerative disorders: NE‐PAL, a pilot randomised controlled study. BMJ Support Palliat Care 2017;7(2):164–172. [DOI] [PubMed] [Google Scholar]
- 31. Frazzitta G, Maestri R, Bertotti G, et al. Intensive rehabilitation treatment in early Parkinson's disease: a randomized pilot study with a 2‐year follow‐up. Neurorehabil Neural Repair 2015;29(2):123–131. [DOI] [PubMed] [Google Scholar]
- 32.Official Statistics of Finland: Life table by age, year, information and sex. http://pxnet2.stat.fi/PXWeb/pxweb/en/StatFin/StatFin__vrm__kuol/statfin_kuol_pxt_007.px/table/tableViewLayout1/. Accessed October 20, 2019.
- 33. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
- 34.Official Statistics of Finland: Life expectancy at birth by year, information and sex. http://pxnet2.stat.fi/PXWeb/pxweb/en/StatFin/StatFin__vrm__kuol/statfin_kuol_pxt_006.px/table/tableViewLayout1/. Accessed October 20, 2019.
- 35.World Health Organization: Noncommunicable diseases and mental health. https://www.who.int/. https://www.who.int/nmh/events/ncd_action_plan/en/. Accessed October 20, 2019.
- 36.Official Statistics of Finland. Causes of death. Helsinki: Statistics Finland. http://www.stat.fi/til/ksyyt/2016/ksyyt_2016_2017-12-29_kat_002_en.html. Accessed October 20, 2019.
- 37. Kompoliti K, Adler CH, Raman R, et al. Gender and pramipexole effects on levodopa pharmacokinetics and pharmacodynamics. Neurology 2002;58(9):1418–1422. [DOI] [PubMed] [Google Scholar]
- 38. Kumagai T, Nagayama H, Ota T, Nishiyama Y, Mishina M, Ueda M. Sex differences in the pharmacokinetics of levodopa in elderly patients with Parkinson disease. Clin Neuropharmacol 2014;37(6):173–176. [DOI] [PubMed] [Google Scholar]
- 39. Chan AK, McGovern RA, Brown LT, et al. Disparities in access to deep brain stimulation surgery for Parkinson disease: interaction between African American race and Medicaid use. JAMA Neurol 2014;71(3):291–299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. The percentage of patients with unchanged PD diagnoses over a 2‐year follow‐up period (2011–2013) according to sex.
Supplementary Table S2. The number and relative proportion of patients with PD using levodopa and dopamine agonists according to sex and year of diagnosis.


