Abstract
Background
Several immune-related adverse events (irAEs) are reported to be associated with therapeutic efficacy of immune checkpoint inhibitors, yet whether pituitary dysfunction, a life-threatening irAE, affects overall survival (OS) in patients with malignancies is unclear. This prospective study examined the association of pituitary dysfunction (pituitary-irAE) with OS of patients with non-small cell lung carcinoma (NSCLC) or malignant melanoma (MM).
Methods
A total of 174 patients (NSCLC, 108; MM, 66) treated with ipilimumab, nivolumab, pembrolizumab, or atezolizumab at Nagoya University Hospital were evaluated for OS and the development of pituitary-irAE. Kaplan-Meier curves of OS as a function of the development of pituitary-irAE were produced with the log-rank test as a primary endpoint.
Results
Pituitary-irAE was observed in 16 patients (4 (3.7%) with NSCLC, 12 (18.2%) with MM) having two different disease types: hypophysitis with deficiency of multiple anterior pituitary hormones accompanied by pituitary enlargement, and isolated adrenocorticotropic hormone (ACTH) deficiency without pituitary enlargement. Among these patients, 6 developed pituitary-irAE while being treated with ipilimumab (6/25 patients (24.0%) treated with ipilimumab) and 10 developed pituitary-irAE during treatment with nivolumab or pembrolizumab (10/167 (6.0%)). All 16 patients had ACTH deficiency and were treated with physiological doses of hydrocortisone. The development of pituitary-irAE was associated with better OS in patients with NSCLC (not reached vs 441 (95% CI not calculated) days, p<0.05) and MM (885 (95% CI 434 to 1336) vs 298 (95% CI 84 to 512) days, p<0.05).
Conclusions
In our study cohort, the incidence of pituitary-irAE was higher than previously reported and the development of pituitary-irAE predicted better prognosis for both NSCLC and MM when patients were treated with physiological doses of hydrocortisone.
Clinical trials registration
UMIN000019024.
Keywords: immunotherapy, melanoma, lung neoplasms
Background
Immune checkpoint inhibitors (ICIs) including antibodies against anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and anti-programmed cell death-1 (PD-1) have recently emerged as promising treatments for advanced malignancies.1 However, ICIs can cause adverse events, termed immune-related adverse events (irAEs), including pneumonitis, skin toxicities, colitis, and endocrine dysfunction.2 3
ICIs were reported to have better antitumor efficacy in patients who developed some irAEs.4–9 In terms of the relationship of irAE to overall survival (OS), several studies reported that skin-irAE is associated with better OS in both patients with malignant melanoma (MM) and non-small cell lung carcinoma (NSCLC),4 8 10 11 whereas another study found that OS of patients with NSCLC was similar between those who developed skin-irAE and those who did not.7 The different outcomes of these previous clinical trials and retrospective studies could be due to the different study designs including evaluation methods and definition of irAEs.
Endocrine-irAEs comprise pituitary dysfunction, adrenal insufficiency, thyroid dysfunction, hypoparathyroidism, and type 1 diabetes mellitus (T1DM).12 13 Among them, thyroid-irAE induced by an anti-PD-1 antibody (aPD-1 Ab) was reported to be associated with better OS in patients with NSCLC in a phase I clinical trial14 and retrospective studies.5 15 16 In contrast, thyroid-irAE induced by aPD-1 Ab was not associated with better OS of patients with MM,16 suggesting that the association of thyroid-irAE with improved outcomes varied among patients with NSCLC or MM. On the other hand, pituitary-irAE is almost always accompanied by adrenocorticotropic hormone (ACTH) deficiency,17 a life-threatening disorder, and a retrospective study reported that pituitary dysfunction induced by ipilimumab was associated with better OS in patients with MM.18 However, symptoms of ACTH deficiency, such as fatigue, appetite loss, and weight loss, frequently occur in patients with malignancies even if they do not develop pituitary-irAE. As such, pituitary-irAE could be overlooked when pituitary hormones are not measured regularly, and its incidence may not have been accurately reflected in previous studies.
To clarify the association of pituitary-irAE with OS in patients with NSCLC or MM, we performed a prospective study in which endocrine-irAEs induced by ICIs were set as a primary endpoint.
Methods
Patients
To clarify the clinical features of endocrine-irAEs, we conducted a prospective study to analyze irAEs in patients treated with ICIs since November 2, 2015 (UMIN000019024). All patients with NSCLC or MM who started ICI treatment between November 2, 2015 and August 30, 2019 at Nagoya University Hospital were included in this study (figure 1). Patients who had more than two malignancies were excluded. Written informed consent was obtained from all patients. Ipilimumab was administered at 3 mg/kg every 3 weeks for 4 cycles. Nivolumab was administered at 3 mg/kg or 240 mg every 2 weeks, with the exception of some patients with MM, who were treated with 2 mg/kg every 3 weeks. Pembrolizumab was administered at 2 mg/kg or 200 mg every 3 weeks. Atezolizumab was administered at 1200 mg every 3 weeks. All ICI treatment was continued until disease progressed, death, or unacceptable severe adverse events occurred, or if patients withdrew consent for treatment.
Figure 1 .
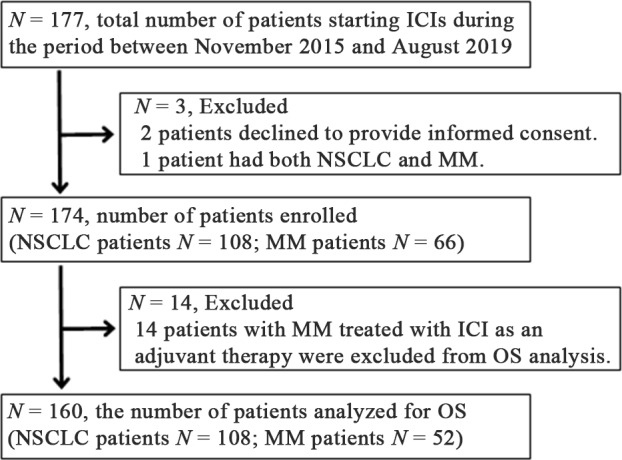
Enrollment of study subjects. ICI, immune checkpoint inhibitor; MM, malignant melanoma; NSCLC, non-small cell lung carcinoma; OS, overall survival.
Assessments
To examine endocrine-irAEs, ACTH, cortisol, free T3 (FT3), free T4 (FT4), thyroid-stimulating hormone (TSH), and blood glucose levels were assessed at baseline and every 6 weeks after the first administration of ICI for 24 weeks as previously described.19 After the initial 24 weeks, pituitary hormones were measured if clinically needed until the visits stopped. Each endocrine-irAE was defined according to the Japan Endocrine Society clinical guidelines for endocrine-irAEs.12 The cut-off for the diagnosis of ACTH deficiency was decreased peak serum cortisol value (<18 µg/dL) and impaired responses of ACTH (<2-fold of baseline) in corticotropin-releasing hormone loading tests (intravenous injection with 100 µg human corticorelin),20 which were performed in the morning. The frequency of hyponatremia, defined as a serum sodium concentration <135 mEq/L, at the onset of pituitary-irAE was compared with that for patients who did not develop pituitary-irAE. In the latter group, the frequency was evaluated around the median time of onset of pituitary-irAE induced by each ICI (ipilimumab, 71 days; nivolumab, 171 days; pembrolizumab, 127 days). OS was determined for participants in this study until death from any cause. Patients with MM were excluded from the OS analysis if they were treated with ICIs as an adjuvant therapy (figure 1). Non-endocrine-irAEs, as reported in the guideline,13 were diagnosed by attending physicians and subjected to analysis if the grade was higher than 1. All irAEs were monitored and graded using CTCAE 5.0 criteria. Fifty-eight patients treated with nivolumab and 78 patients treated with pembrolizumab were included in our previous study that analyzed the incidence of thyroid dysfunction.19 21
Statistical analysis
Continuous variables of patient characteristics (eg, age, days to diagnosis) are expressed as means±SD or median and IQR. Differences among continuous variables were tested for significance with the two-sample t-test. Values of nominal variables were compared using Fisher’s exact test. OS was analyzed using the Kaplan-Meier method, expressed as median and 95% CI and compared using a log-rank test. All statistical tests were two sided, and significance was defined as a p value of <0.05. All statistical analyses were performed with IBM SPSS Statistics V.25.
Results
Patient characteristics
A total of 174 patients with either NSCLC or MM who started ipilimumab, nivolumab, pembrolizumab, or atezolizumab therapy were enrolled in this study (figure 1). Clinical variables for patients with NSCLC and MM are presented in table 1. IrAEs occurred in 34/108 patients (31.5%) with NSCLC and 38/66 patients (57.6%) with MM (table 1). Endocrine-irAEs including pituitary-irAE, thyroid-irAE, and T1DM occurred in 13/108 (12.0%) patients with NSCLC and 20/66 (30.3%) patients with MM. The number of each type of irAE observed, including pituitary-irAE, thyroid-irAE, T1DM, interstitial pneumonitis (lung-irAE), skin toxicities (skin-irAE), and gastrointestinal toxicities (GI-irAE), is shown in table 1. No cases were treated with a reduced dose of ICI due to the development of irAEs.
Table 1.
Clinical characteristics and number of each irAE type among patients with NSCLC and MM
| NSCLC | MM | |
| Characteristic | n=108 | n=66 |
| Age, years | 67±10 | 69±12 |
| Sex | ||
| Female | 29 (26.9%) | 27 (40.9%) |
| Male | 79 (73.1%) | 39 (59.1%) |
| Drugs | ||
| Ipi | 0 | 24 (36.4%) |
| Niv | 57 (52.8%) | 34 (51.5%) |
| Pem | 51 (47.2%) | 30 (45.5%) |
| Ipi+Niv | 0 | 1 (1.5%) |
| Ate | 9 (8.3%) | 0 |
| History of prior ICI use | 0 | 12 (18.2%) |
| Treatment lines | ||
| Adjuvant | 0 | 14 (21.2%) |
| First line | 32 (29.6%) | 33 (50.0%) |
| ≥Second line | 76 (70.4%) | 19 (28.8%) |
| Follow-up, days | 396±304 | 343±319 |
| Total no. of patients who developed irAEs | 34 (31.5%) | 38 (57.6%) |
| Pituitary-irAE | 4 (3.7%) | 12 (18.2%) |
| Thyroid-irAE | 8 (7.4%) | 11 (16.7%) |
| T1DM | 2 (1.9%) | 1 (1.5%) |
| Lung-irAE | 13 (12.0%) | 6 (9.1%) |
| Skin-irAE | 5 (4.6%) | 4 (6.1%) |
| GI-irAE | 4 (3.7%) | 12 (18.2%) |
| Other | 3 (2.8%) | 4 (6.1%) |
Data are mean±SD or n (%).
Ate, atezolizumab; GI-irAE, gastrointestinal irAE; ICI, immune checkpoint inhibitor; Ipi, ipilimumab; irAE, immune-related adverse event; MM, malignant melanoma; Niv, nivolumab; NSCLC, non-small cell lung carcinoma; Pem, pembrolizumab; T1DM, type 1 diabetes mellitus.
Pituitary dysfunction induced by ICIs
Pituitary-irAE occurred in 6/25 patients (24.0%) during treatment with ipilimumab, 10/167 patients (6.0%) during treatment with nivolumab or pembrolizumab. Pituitary-irAE did not occur during treatment with atezolizumab (0/9). Pituitary-irAE induced by aPD-1 Ab, nivolumab, or pembrolizumab was observed in 6/59 patients with MM and 4/108 patients with NSCLC. There was no difference in the frequency of pituitary-irAE induced by aPD-1 Ab between patients with MM and those with NSCLC (10.2% (6/59) in patients with MM vs 3.7% (4/108) in patients with NSCLC, p=0.168). Among the 16 patients who developed pituitary-irAE, three demonstrated clinical characteristics of hypophysitis such as pituitary enlargement and deficiencies in multiple anterior pituitary hormones including ACTH (online supplementary table S1). These three patients presented with headache as an initial symptom that could be caused by enlargement of the pituitary glands at irAE onset. A representative MRI showing an enlargement of the pituitary gland at hypophysitis onset is shown in figure 2A. This enlargement was completely resolved at 6 months after onset (figure 2B) and did not change during a 1-year follow-up (data not shown). The other 13 patients developed isolated ACTH deficiency (IAD), but none exhibited enlarged pituitary glands at IAD onset (online supplementary table S1 and figure 2C). Re-evaluation of pituitary gland size 6 months and/or 1 year after IAD onset for 11 of these patients showed no change in size for seven patients (representative MRI image, figure 2D) and a slightly decreased size for the other four patients. In terms of clinical characteristics of patients who developed hypophysitis and IAD, the usage rate of aCTLA-4 Ab and incidence of pituitary enlargement were significantly higher for patients who developed hypophysitis than those who had IAD (table 2). The time to diagnosis of pituitary-irAE after the first ICI administration was significantly shorter in patients with hypophysitis compared with those with IAD (table 2). There were no significant differences in the other clinical variables we examined (eg, malignancy type, age, sex, and history of prior immunotherapy) (table 2). ACTH deficiency was observed in all cases with pituitary-irAE (online supplementary table S2), and these patients were treated with physiological doses of hydrocortisone (10–20 mg/day). ICI was re-administered with the same dose initially used after general conditions were stabilized by hormone replacement therapy. During the observation period of this study, no patients discontinued hormone replacement therapies (hydrocortisone and/or levothyroxine). The frequency of hyponatremia was significantly higher in patients who developed pituitary-irAE than those who did not (6/16 (37.5%) vs 10/120 (8.3%), p<0.01).
Figure 2 .
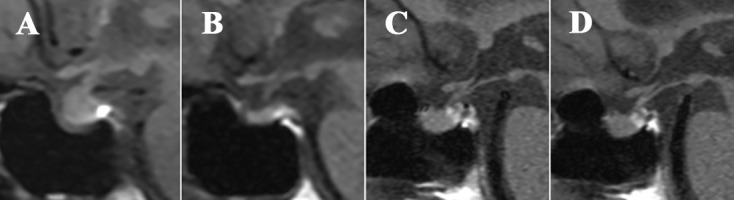
MRI of the pituitary gland in a patient with pituitary immune-related adverse event (pituitary-irAE). Representative MRI for a patient (Ipi001) who developed hypophysitis shows that the pituitary gland was enlarged at pituitary-irAE onset (A) but had returned to normal size 6 months after the onset (B). Representative MRI for a patient (Niv096) who developed isolated adrenocorticotropic hormone deficiency show a normal-sized pituitary gland at pituitary-irAE onset (C) and absence of changes at 6 months after onset (D).
Table 2.
Clinical characteristics of patients who developed pituitary-irAE
| Hypophysitis n=3 |
IAD n=13 |
P value | |
| Malignancy | 0.529 | ||
| NSCLC | 0 | 4 (30.8%) | |
| MM | 3 (100%) | 9 (69.2%) | |
| Age, years | 59±11 | 67±10 | 0.251 |
| Sex | 0.509 | ||
| Female | 0 | 5 (38.5%) | |
| Male | 3 (100%) | 8 (61.5%) | |
| History of prior immunotherapy | 2 (66.7%) | 3 (23.1%) | 0.214 |
| Latest ICI | 0.036 | ||
| aCTLA-4 Ab | 3 (100%) | 3 (23.1%) | |
| aPD-1 Ab | 0 | 10 (76.9%) | |
| Days to diagnosis from the first administration of the latest drug | 56±27 | 162±108 | 0.008 |
| No. of cases with enlarged pituitary by MRI | 3 (100%) | 0 | 0.002 |
Data are n (%) or mean±SD.
aCTLA-4 Ab, anti-cytotoxic T-lymphocyte antigen 4 antibodies; aPD-1 Ab, anti-programmed cell death-1 antibodies; IAD, isolated adrenocorticotropic hormone deficiency; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; MM, malignant melanoma; NSCLC, non-small cell lung carcinoma.
jitc-2020-000779supp001.pdf (69.4KB, pdf)
Thyroid dysfunction induced by ICIs
Thyroid-irAE occurred in 1/25 patients (4.0%) during treatment with ipilimumab and in 18/167 patients (10.8%) during treatment with nivolumab or pembrolizumab. The incidence of thyroid-irAE tended to be higher in patients treated with aPD-1 Ab than those who received aCTLA-4 Ab, although the difference was not significant (18/167 (10.8%) vs 1/25 (4.0%), p=0.477). The number of patients who developed destructive thyroiditis, hypothyroidism, and hyperthyroidism was 13, 5, and 1, respectively. All five patients who developed hypothyroidism and 12/13 who developed destructive thyroiditis required hormone therapy with levothyroxine.
Patients who developed any irAE had longer OS than those without irAEs
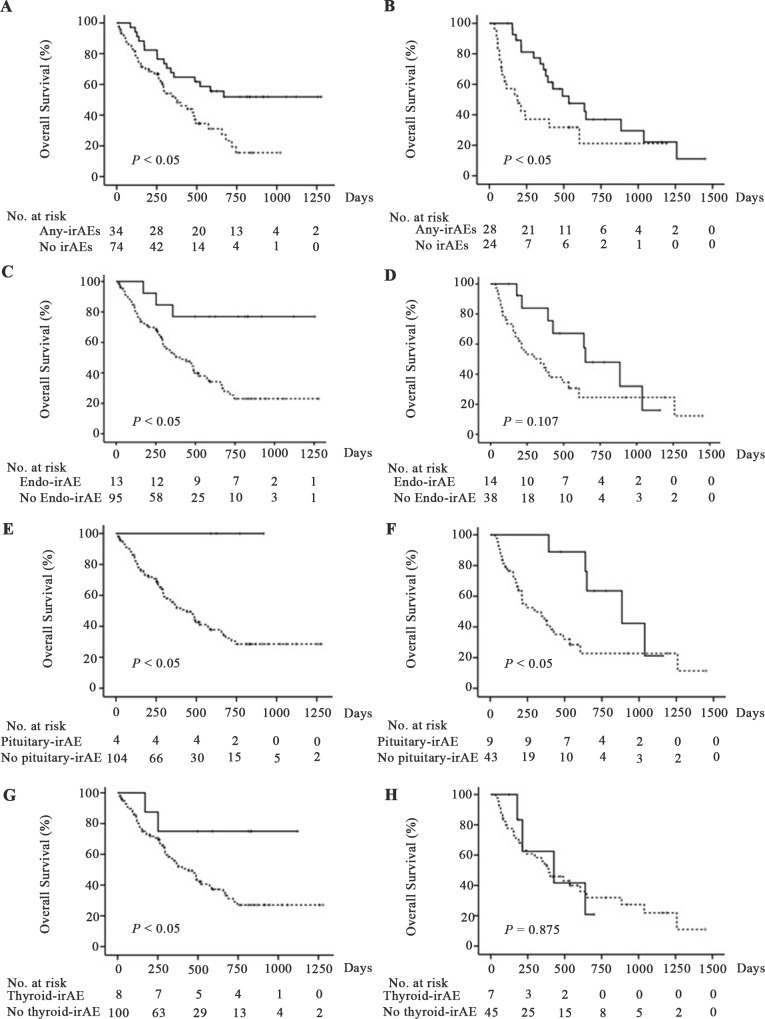
OS was significantly longer for both patients with NSCLC and MM who developed any irAE than those without irAEs (NSCLC: not reached vs 378 (95% CI 220 to 536) days, p<0.01, figure 3A, online supplementary table S3; MM: 534 (95% CI 245 to 823) vs 189 (95% CI 52 to 326) days, p<0.05, figure 3B, online supplementary table S3). To examine the contribution of endocrine-irAEs to increased OS, 13 patients with NSCLC and 14 patients with MM who developed endocrine-irAEs were included in OS analysis. OS was significantly longer for patients with NSCLC who developed endocrine-irAEs than those who did not (not reached vs 401 days (95% CI 240 to 562), p<0.01, figure 3C, online supplementary table S3). In patients with MM, there were no significant differences in OS between patients who developed endocrine-irAEs and those who did not (649 days (95% CI 367 to 931) vs 342 days (95% CI 131 to 553), p=0.107, figure 3D, online supplementary table S3).
Figure 3 .
Overall survival (OS) after initiation of immune checkpoint inhibitor treatment in patients with and without immune-related adverse events (irAEs). OS of patients with non-small cell lung carcinoma (NSCLC) (A, C, E, G) or malignant melanoma (MM) (B, D, F, H). Solid and dashed lines indicate OS of patients who did and did not develop each irAE, respectively. The OS was significantly prolonged for patients who developed any irAEs than those who did not for both patients with NSCLC and MM (A, B). The OS was significantly prolonged for patients who developed endocrine-irAEs than those who did not for patients with NSCLC (C) but not patients with MM (D). Among patients with NSCLC and those with MM, the OS was significantly prolonged for patients who developed pituitary-irAE than those who did not (E, F). The OS was significantly prolonged for patients who developed thyroid-irAE than those who did not for patients with NSCLC (G) but not patients with MM (H).
Pituitary-irAE was associated with prolonged OS in patients with NSCLC or MM
Four patients with NSCLC and nine patients with MM with pituitary-irAE were included in the analysis of OS. OS was significantly longer for both patients with NSCLC and MM who developed pituitary-irAE than those who did not (NSCLC: not reached vs 441 days (95% CI not calculated), p<0.05, figure 3E, online supplementary table S3; MM: 885 days (95% CI 434 to 1336) vs 298 says (95% CI 84 to 512), p<0.05, figure 3F, online supplementary table S3). There were no significant differences between patients with NSCLC (online supplementary table S4) or MM (online supplementary table S5) who developed pituitary-irAE and those who did not in terms of sex, drugs used, prior history of other ICIs, and treatment lines, although the mean ages were significantly younger and the ipilimumab usage rate was significantly higher for patients with MM who developed pituitary-irAE compared with those who did not (online supplementary table S5). The analysis of OS included patients treated with ICI monotherapy as well as sequential use of several ICIs because the number of patients who were treated with only one ICI during the study period was too small (online supplementary table S6). There were no significant differences in the usage rate of multiple ICIs between patients who developed pituitary-irAE for patients with either NSCLC (0/4 with pituitary-irAE vs 8/104 without pituitary-irAE. p=1.000) or MM (5/9 with pituitary-irAE vs 20/43 without pituitary-irAE. p=0.722). There were no differences in the number of cycles of ICI between patients who developed pituitary-irAE and those who did not, except for patients with MM treated with nivolumab (online supplementary tables S7 and S8). In this group, the number of cycles was significantly higher in the pituitary-irAE group (online supplementary table S8). The time of follow-up was significantly longer for the patients with NSCLC and MM who developed pituitary-irAE than those who did not (online supplementary tables S7 and S8).
Thyroid-irAE was associated with prolonged OS in patients with NSCLC, but not MM
OS was significantly longer for patients with NSCLC who developed thyroid-irAE than those who did not (not reached vs 441 days (95% CI 317 to 565), p<0.05, figure 3G, online supplementary table S3). In contrast, patients with MM who developed thyroid-irAE and those who did not had similar OS (427 days (95% CI 0 to 871) vs 393 days (95% CI 219 to 567), p=0.875, figure 3H, online supplementary table S3). There were no significant differences between patients with NSCLC (online supplementary table S9) or MM (online supplementary table S10) who developed thyroid-irAE and those who did not for other clinical factors including sex, drugs used, prior history of other ICIs, and treatment lines. Moreover, there were no significant differences in the usage rate of multiple ICIs between patients who developed thyroid-irAE and those who did not among patients with NSCLC (0/8 with thyroid-irAE vs 8/100 without thyroid-irAE; p=1.000) or MM (2/7 with thyroid-irAE vs 24/45 without thyroid-irAE; p=0.419).
OS was not associated with other types of irAE in patients with NSCLC or MM
Although patients who had any irAE showed longer OS than those without irAEs, the development of non-endocrine-irAEs was not associated with longer OS in patients with either NSCLC or MM (NSCLC: 585 days (95% CI 321 to 849) vs 441 days (95% CI 297 to 585), p=0.247, online supplementary figure 1A, table S3; MM: 534 days (95% CI 268 to 800) vs 241 days (95% CI 0 to 523), p=0.268, online supplementary figure 1B, table S3). As for each non-endocrine-irAE, there were no differences in OS between patients who developed lung-irAE, skin-irAE, or GI-irAE and those who did not for patients with NSCLC (lung-irAE, 488 days (95% CI 167 to 809) vs 474 days (95% CI 334 to 614), p=0.710; skin-irAE, 669 days (95% CI 438 to 900) vs 441 days (95% CI 310 to 572), p=0.550; GI-irAE, not reached vs 481 days (95% CI 351 to 597), p=0.731) or MM (lung-irAE, 1259 days (95% CI 0 to 2655) vs 393 days (95% CI 237 to 549), p=0.202; skin-irAE, 649 days (95% CI not calculated) vs 379 (95% CI 252 to 506), p=0.560; GI-irAE, 491 days (95% CI 152 to 830) vs 393 days (95% CI 174 to 612), p=0.581) (online supplementary figure 1C‒H, table S3). For skin-irAEs, exanthema and erythema, but not vitiligo, were observed in this study.
jitc-2020-000779supp002.pdf (479.3KB, pdf)
Discussion
This prospective study, in which all patients with pituitary-irAE were treated with physiological doses of hydrocortisone, revealed that pituitary-irAE was associated with prolonged OS in patients with MM or NSCLC. Furthermore, our study confirmed the findings of previous retrospective studies and a phase I trial that thyroid-irAE was associated with prolonged OS in patients with NSCLC but not those with MM. Thus, the outcome of pituitary-irAEs and thyroid-irAEs in terms of OS differed between NSCLC and MM.
The results of this prospective study clearly demonstrated that the incidence of pituitary-irAE was higher (24.0% and 6.0% for patients treated with aCTLA-4 Ab and aPD-1 Ab, respectively) than previously reported.17 22–24 In a retrospective analysis of a medical record database, Faje et al reported that the incidence of pituitary dysfunction induced by aCTLA-4 Ab and aPD-1 Ab was 13.6% or 0.5%, respectively.25 Pituitary-irAE could have been overlooked in that study because most symptoms associated with pituitary-irAE such as fatigue, appetite loss, and weight loss also occur in patients with malignancies. One strength of the present prospective study is that pituitary-irAE was far more likely to be detected because of the scheduled endocrine evaluation during the fixed period regardless of whether ICIs were continued. In addition, this study has less selection bias than clinical trials because all patients treated with ICIs in our hospital were prospectively included. Thus, the incidence of pituitary-irAE observed in this study is more likely to reflect that which occurs in a real-world clinical practice.
Concerning the association of pituitary-irAE with OS, only one retrospective study that analyzed a clinical record database reported an association of pituitary dysfunction induced by a CTLA-4 Ab (ipilimumab) with prolonged OS in patients with MM18 and no prior studies have shown an association of pituitary-irAE with OS in patients with NSCLC. It is also unclear whether pituitary dysfunction induced by aPD-1 Ab is associated with OS. This study analyzed the association of OS with pituitary dysfunction induced by aCTLA-4 Ab or aPD-1 Ab, and clarified that pituitary-irAE is associated with prolonged OS in patients with MM and also those with NSCLC. Thus, pituitary-irAE could be a potential biomarker to predict better outcome of ICI treatments for these types of malignancies, although it sometimes develops several months after the initial administration of ICIs.
Given the contribution of pituitary-irAE to better OS, it is important to understand its clinical characteristics, how to manage them appropriately, and why pituitary-irAE is associated with prolonged survival. This study clearly demonstrated that ICI can induce two types of pituitary dysfunction: hypophysitis, associated with deficiencies in multiple pituitary hormones accompanied by pituitary enlargement, and IAD without pituitary enlargement. Our data also showed that (1) ACTH deficiency was observed in all cases, (2) IAD can be induced by either aCTLA-4 Ab or aPD-1 Ab, and (3) hypophysitis was induced only by aCTLA-4 Ab.
The mechanisms underlying the association of pituitary-irAE with better outcome of ICI treatments are still unknown. The development of vitiligo was associated with better tumor response in patients with MM,10 suggesting that melanoma cells and normal melanocytes may have shared antigens. It would be interesting to explore whether there are shared antigens between pituitary cells and MM or NSCLC cells to determine if they are involved in development of pituitary-irAE. Previous studies proposed that complement activation following aCTLA-4 Ab binding to CTLA-4 expressed on pituitary cells is involved in pathogenesis of hypophysitis induced by aCTLA-4 Ab in mice26 and humans.17 Given that aCTLA-4 Ab is an IgG1 and aPD-1 Ab is an IgG4 that cannot activate complement, the mechanism underlying pituitary-irAEs likely differs among ICIs.
Determining effective treatment strategies for pituitary dysfunction induced by ICIs is critical. Although the effect of high-dose glucocorticoids to treat pituitary-irAE has not been fully examined, retrospective studies reported that such doses did not affect recovery of pituitary function27 and instead shortened OS.18 In this study, all patients who developed pituitary-irAE were treated with physiological doses of hydrocortisone. In addition, all patients who developed pituitary-irAE were re-administered ICIs after their general conditions were stabilized by hormone treatment. Although pituitary-irAE can be life threatening,28 29 this study demonstrated the association of pituitary-irAE with better OS when patients are treated with physiological doses of hydrocortisone.
Although hyponatremia can accompany adrenal insufficiency, some patients with cancer also often develop hyponatremia30 due to inappropriate anti-diuretic hormone secretion,31 gastrointestinal or renal losses and/or diminished sodium intake, use of diuretics, hypervolemic state, renal failure, or intake of hypotonic fluids.32 33 In this study, we first demonstrated that the frequency of hyponatremia was significantly higher in the patients who developed pituitary-irAE compared with those who did not. These data suggest that ACTH and cortisol levels in the blood should be measured to detect development of pituitary-irAE when hyponatremia is observed in patients treated with ICIs.
Previous studies reported an association between better OS with irAEs other than endocrine-irAEs, including skin-irAE or lung-irAE.4 7 8 10 11 We clearly demonstrated the association of pituitary-irAE with better ICI treatment outcomes, yet no irAE type other than endocrine-irAEs was associated with better OS. Endocrine-irAEs were definitely determined as primary outcomes according to Japan Endocrine Society clinical guidelines,12 whereas other irAEs were diagnosed by attending physicians in practice, which may have affected the results of this study.
This study has some limitations. First, analysis of OS for each ICI regimen did not yield statistically significant results likely due to small sample sizes. We therefore included both patients treated with ICI monotherapy as well as those treated with several ICIs sequentially in the analyses. Second, to calculate the incidence of pituitary dysfunction, we defined the drug that was in use at onset of pituitary dysfunction to be the causative drug, and we cannot exclude the possibility that a previously used ICI may have affected the development of irAEs. Third, there may be a bias in that pituitary-irAE can be observed in patients who survived for a longer time. Actually, some patients showed late onset of pituitary-irAE in this study. Additional studies that include landmark analyses for larger study populations are needed to address these limitations.
Conclusions
Our prospective study clarified the exact incidence of pituitary-irAE in a real-world clinical practice and the association of pituitary-irAE accompanied by ACTH deficiency with a better outcome of ICI treatments in both NSCLC and MM when patients were treated with physiological doses of hydrocortisone. Our findings indicate that we should measure pituitary hormones when pituitary-irAE is suspected based on hyponatremia, so that ACTH deficiency in patients treated with ICI will not be overlooked.
Footnotes
Contributors: SI designed the study. TK and SI performed the clinical study. TK, SI, and HA analyzed the data. All authors treated the patients who were enrolled, collected and discussed the data. TK, SI, and HA wrote the manuscript. All authors were involved in revising the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SI receives personal fees from Ono Pharmaceutical Company, Bristol-Myers Squibb, MSD K.K., and Chugai Pharmaceutical Co., Ltd. TO receives personal fees from MSD K.K. HT receives grants from MSD K.K. YI receives grants from Sanwa Kagaku Kenkyusho, Kowa Pharmaceutical, MSD K.K., Dainippon Sumitomo, Kyowa Kirin Co., Ltd., Chugai Pharmaceutical Co., Ltd., Boehringer Ingelheim, Nihon Medi-Physics Co., Ltd., and personal fees from Astellas Pharma, Daiichi Sankyo, and Ono Pharmaceutical Company. KY receives personal fees from Ono Pharmaceutical Company, MSD K.K., and Bristol-Myers Squibb. TH received personal fees from Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Company, and Bristol-Myers Squibb while the study was being conducted. TH also received grants from Boehringer Ingelheim, Taiho Pharmaceutical Co., Ltd., and personal fees from Boehringer Ingelheim outside the submitted work. MMS receives personal fees from Ono Pharmaceutical Company, Bristol-Myers Squibb, MSD K.K., and Chugai Pharmaceutical Co., Ltd. MA receives grants from Kyowa Kirin Co., Ltd. YA receives grants and personal fees from Ono Pharmaceutical Company, Chugai Pharmaceutical Co., Ltd., and Taiho Pharmaceutical Co. Ltd. as well as personal fees from Bristol-Myers Squibb. MA receives grants and personal fees from Ono Pharmaceutical Company, MSD K.K., and personal fees from Bristol-Myers Squibb. YH receives grants and personal fees from Ono Pharmaceutical Company, Chugai Pharmaceutical Co., Ltd., Eli Lilly, Boehringer Ingelheim, Bristol-Myers Squibb, MSD K.K., and Pfizer; grants from Novartis and Taiho Pharmaceutical Co., Ltd., and personal fees from Astra Zeneca. HA receives grants from Ono Pharmaceutical Company, MSD K.K., Chugai Pharmaceutical Co. Ltd., and personal fees from Ono Pharmaceutical Company, Bristol-Myers Squibb, and MSD K.K.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Ethical Committee of Nagoya University Hospital, No. 2015-0273. Written informed consent was obtained from all patients.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
References
- 1. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. JCO 2015;33:1974–82. 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scott LJ. Nivolumab: a review in advanced melanoma. Drugs 2015;75:1413–24. 10.1007/s40265-015-0442-6 [DOI] [PubMed] [Google Scholar]
- 3. Graziani G, Tentori L, Navarra P. Ipilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol Res 2012;65:9–22. 10.1016/j.phrs.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 4. Freeman-Keller M, Kim Y, Cronin H, et al. . Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–94. 10.1158/1078-0432.CCR-15-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grangeon M, Tomasini P, Chaleat S, et al. . Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer 2019;20:201–7. 10.1016/j.cllc.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 6. Indini A, Di Guardo L, Cimminiello C, et al. . Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 2019;145:511–21. 10.1007/s00432-018-2819-x [DOI] [PubMed] [Google Scholar]
- 7. Ricciuti B, Genova C, De Giglio A, et al. . Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 2019;145:479–85. 10.1007/s00432-018-2805-3 [DOI] [PubMed] [Google Scholar]
- 8. Haratani K, Hayashi H, Chiba Y, et al. . Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018;4:374–8. 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okada N, Kawazoe H, Takechi K, et al. . Association between immune-related adverse events and clinical efficacy in patients with melanoma treated with nivolumab: a multicenter retrospective study. Clin Ther 2019;41:59–67. 10.1016/j.clinthera.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 10. Teulings H-E, Limpens J, Jansen SN, et al. . Vitiligo-like depigmentation in patients with stage III–IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015;33:773–81. 10.1200/JCO.2014.57.4756 [DOI] [PubMed] [Google Scholar]
- 11. Fujisawa Y, Yoshino K, Otsuka A, et al. . Retrospective study of advanced melanoma patients treated with ipilimumab after nivolumab: analysis of 60 Japanese patients. J Dermatol Sci 2018;89:60–6. 10.1016/j.jdermsci.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 12. Arima H, Iwama S, Inaba H, et al. . Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan Endocrine Society. Endocr J 2019;66:581–6. 10.1507/endocrj.EJ19-0163 [DOI] [PubMed] [Google Scholar]
- 13. Brahmer JR, Lacchetti C, Schneider BJ, et al. . Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osorio JC, Ni A, Chaft JE, et al. . Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583–9. 10.1093/annonc/mdw640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim HI, Kim M, Lee S-H, et al. . Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology 2017;7:e1375642. 10.1080/2162402X.2017.1375642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamauchi I, Yasoda A, Matsumoto S, et al. . Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One 2019;14:e0216954. 10.1371/journal.pone.0216954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caturegli P, Di Dalmazi G, Lombardi M, et al. . Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol 2016;186:3225–35. 10.1016/j.ajpath.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faje AT, Lawrence D, Flaherty K, et al. . High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–14. 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi T, Iwama S, Yasuda Y, et al. . Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: a prospective study. J Endocr Soc 2018;2:241–51. 10.1210/js.2017-00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yanase T, Tajima T, Katabami T, et al. . Diagnosis and treatment of adrenal insufficiency including adrenal crisis: a Japan Endocrine Society clinical practice guideline [Opinion]. Endocr J 2016;63:765–84. 10.1507/endocrj.EJ16-0242 [DOI] [PubMed] [Google Scholar]
- 21. Okada N, Iwama S, Okuji T, et al. . Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer. In Press 2020;122:771–7. 10.1038/s41416-020-0736-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary 2016;19:82–92. 10.1007/s11102-015-0671-4 [DOI] [PubMed] [Google Scholar]
- 23. Corsello SM, Barnabei A, Marchetti P, et al. . Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab 2013;98:1361–75. 10.1210/jc.2012-4075 [DOI] [PubMed] [Google Scholar]
- 24. González-Rodríguez E, Rodríguez-Abreu D, Spanish Group for Cancer Immuno-Biotherapy (GETICA) . Immune checkpoint inhibitors: review and management of endocrine adverse events. Oncologist 2016;21:804–16. 10.1634/theoncologist.2015-0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Faje A, Reynolds K, Zubiri L, et al. . Hypophysitis secondary to nivolumab and pembrolizumab is a clinical entity distinct from ipilimumab-associated hypophysitis. Eur J Endocrinol 2019;181:211–9. 10.1530/EJE-19-0238 [DOI] [PubMed] [Google Scholar]
- 26. Iwama S, De Remigis A, Callahan MK, et al. . Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014;6:230ra245. 10.1126/scitranslmed.3008002 [DOI] [PubMed] [Google Scholar]
- 27. Min L, Hodi FS, Giobbie-Hurder A, et al. . Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res 2015;21:749–55. 10.1158/1078-0432.CCR-14-2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arlt W, Allolio B. Adrenal insufficiency. Lancet 2003;361:1881–93. 10.1016/S0140-6736(03)13492-7 [DOI] [PubMed] [Google Scholar]
- 29. Bergthorsdottir R, Leonsson-Zachrisson M, Odén A, et al. . Premature mortality in patients with Addison's disease: a population-based study. J Clin Endocrinol Metab 2006;91:4849–53. 10.1210/jc.2006-0076 [DOI] [PubMed] [Google Scholar]
- 30. Berardi R, Antonuzzo A, Blasi L, et al. . Practical issues for the management of hyponatremia in oncology. Endocrine 2018;61:158–64. 10.1007/s12020-018-1547-y [DOI] [PubMed] [Google Scholar]
- 31. Raftopoulos H. Diagnosis and management of hyponatremia in cancer patients. Support Care Cancer 2007;15:1341–7. 10.1007/s00520-007-0309-9 [DOI] [PubMed] [Google Scholar]
- 32. Berghmans T, Paesmans M, Body JJ. A prospective study on hyponatraemia in medical cancer patients: epidemiology, aetiology and differential diagnosis. Support Care Cancer 2000;8:192–7. 10.1007/s005200050284 [DOI] [PubMed] [Google Scholar]
- 33. Onitilo AA, Kio E, Doi SAR. Tumor-related hyponatremia. Clin Med Res 2007;5:228–37. 10.3121/cmr.2007.762 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-000779supp001.pdf (69.4KB, pdf)
jitc-2020-000779supp002.pdf (479.3KB, pdf)