Figure 2. Reactivity depends on the structural properties of the assembly.
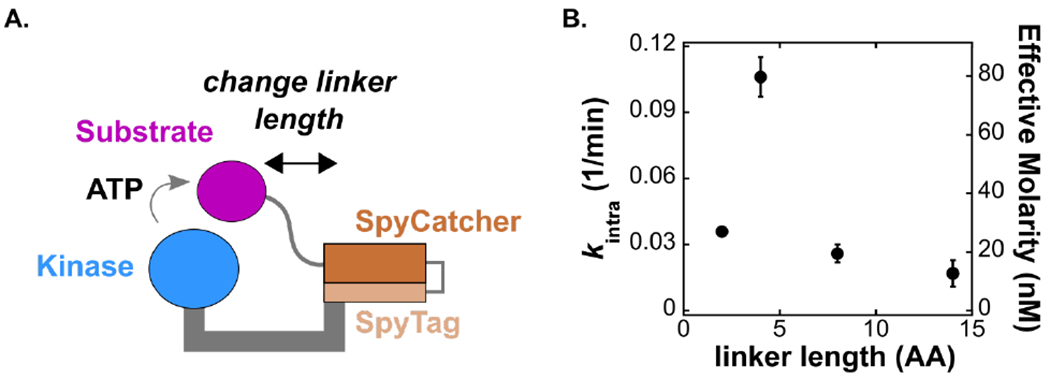
(A) Schematic of the covalently tethered complex. The SpyCatcher-SpyTag complex covalently links the kinase with its substrate. We varied the length of the linker that connects the substrate to SpyCatcher from 2 to 14 residues and measured the unimolecular rate constant (kintra). (B) Plot of kintra vs. number of residues. The secondary y-axis shows the effective molarity for each complex, determined using the bimolecular rate constant from the untethered reaction (Figure 3A). Error bars represent the standard error of kintra obtained from the fit of Vobs vs [tethered complex] as described in Figure S4.
