Figure 1. Co-administration Group Shows Significant Protection from SHIV.CH505 Infection.
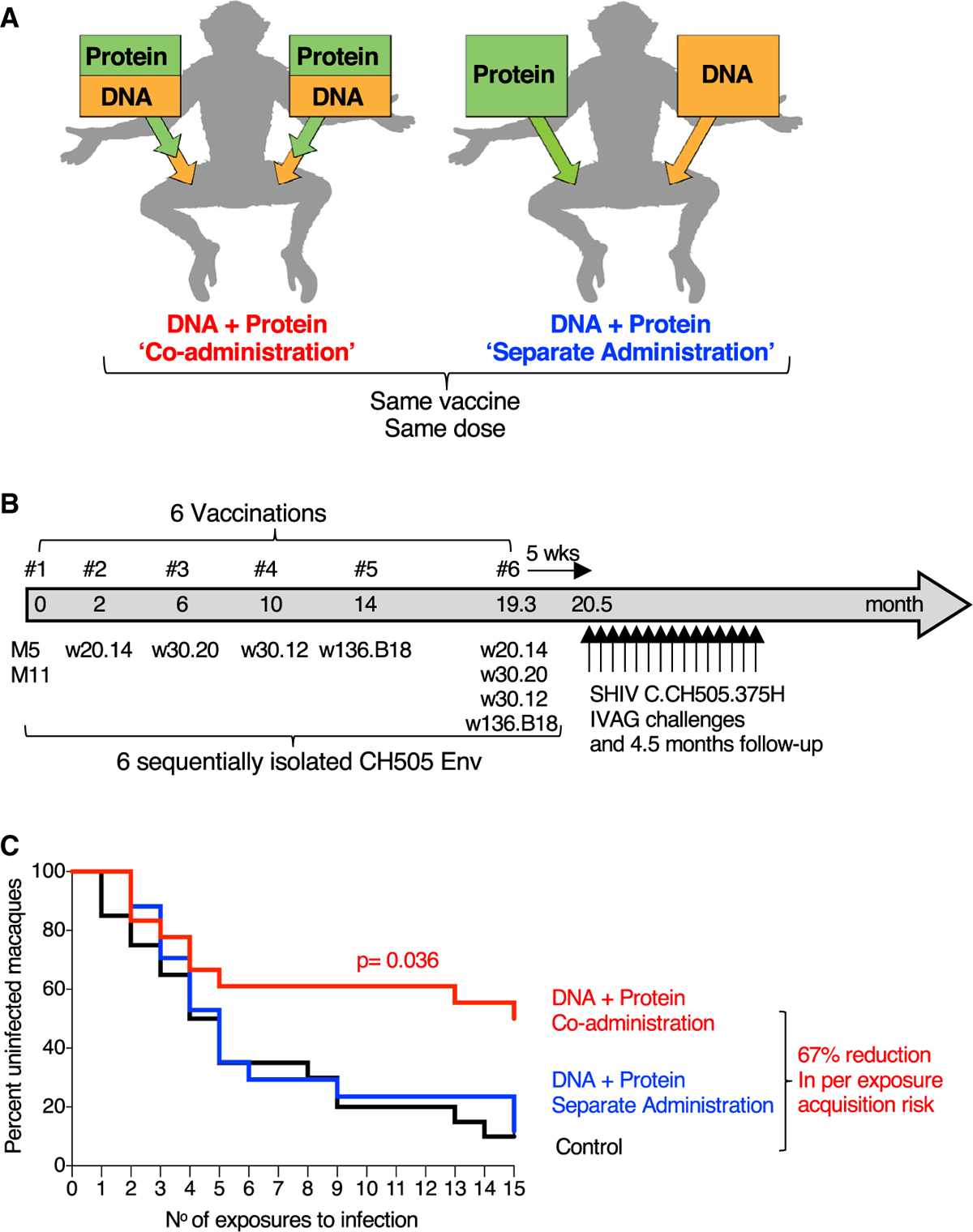
(A) Schematic representation of vaccine delivery of the two components (DNA and protein) in the two vaccination regimens, “Co-administration” in the same anatomical sites and “separate administration” in contralateral sites. The co-administration group received DNA delivered via IM/EP followed immediately by IM injection of the adjuvanted protein. The separate administration group received the vaccine components in different anatomical sites with DNA delivered by IM/EP in the left site and protein IM in the right site. The same vaccine components and the same total vaccine dose were used for both regimens.
(B) Vaccination schedule indicating the sequentially isolated CH505 immunogens used. Five weeks after the last vaccination, the animals were exposed weekly to repeated low-dose vaginal challenges using SHIV.CH505.
(C) Kaplan-Meier curves show the viral acquisition rate after repeated low-dose SHIV.CH505 challenges of the two vaccine groups (n = 18 and 17, respectively) and the control group (n = 20). The RMs were exposed to 15 weekly intravaginal challenges. Infection was defined by two consecutive positive plasma VL measurements. No RMs were censored. p value, exact log-rank test.
