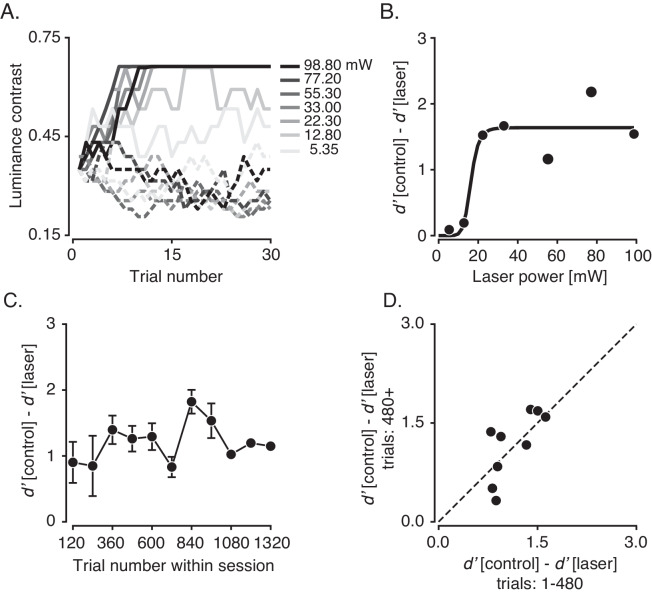
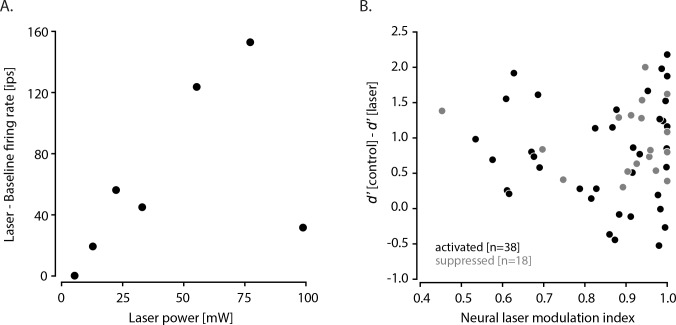
Figure 8. Effect of laser power and repeated optical stimulation on contrast detection.
(A) Contrast values selected by the staircase procedure on laser trials (solid lines) and interleaved control trials (dashed lines) across seven blocks. (B) The difference in d’ between control and laser trials as a function of laser power calculated from the data in (A). A Naka-Rushton fit to the data is shown in black. (C) Differences in d’ between control and laser trials as a function of trial number in each session. Each session consisted of at least five blocks of 120 trials. The duration of an individual trial was 2.80 ± 0.51 s (mean ± SD), and the number of trials per session was 813 ± 253. Points are means and error bars are standard error of the mean (SEM). SEM was not plotted for the final two points, each of which represent data from a single session. (D) Scatter plot of the differences in d’ for early trials (1–480) vs. late trials (480–beyond) within each session.