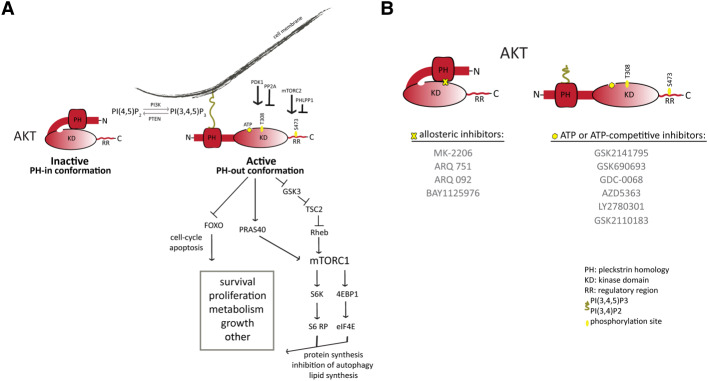
Figure 1. Activation and inhibition of AKT.
(A) PtdIns(3,4,5)P3 (PIP3) is maintained at the cell surface by the opposing function of several class I PI3K kinases and the PTEN phosphatase. AKT remains inactive in the cytoplasm in a PH-in conformation facilitated by interactions of the PH and the kinase domains. Membrane anchored AKT acquires a PH-out conformation, becomes phosphorylated at specific residues by specific kinases and subsequently propagates the signal by phosphorylating different effectors such as PRAS40, GSK3b, FOXO and more. AKT activation increases protein and lipid synthesis, whereas inhibits autophagy and cell apoptosis. (B) Differential mechanism of action of allosteric and ATP-competitive inhibitors of AKT. Allosteric inhibitors lock the PH-in conformation and suppress its membrane localisation and activation, whereas ATP-competitive inhibitors bind to the ATP-pocket of the kinase domain, stabilise the PH-out conformation where AKT becomes phosphorylated and increase its membrane localisation.