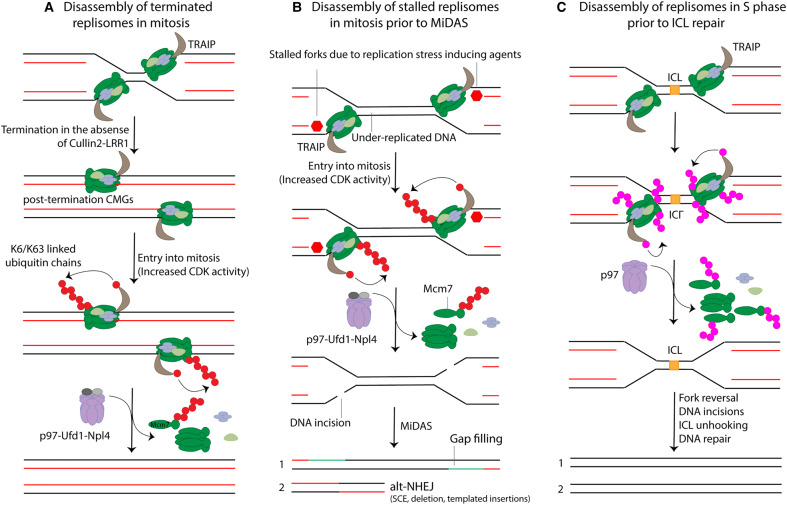
Figure 4. TRAIP-dependent replisome disassembly pathways.
(A) Disassembly of terminated replisomes in mitosis. When termination occurs in the absence of Cullin2LRR1 activity, the terminated replisomes remain associated with chromatin during S phase. Entry into mitosis, driven by an increase in CDK activity, promotes TRAIP-dependent ubiquitylation of Mcm7 within terminated CMGs with K6/K63-linked ubiquitin chains. The ubiquitylated terminated replisome is finally disassembled by p97-Ufd1-Npl4. (B) Disassembly of stalled replisomes in mitosis required for the processing of under-replicated DNA. When DNA replication proceeds in the presence of replication stress-inducing agents such as aphidicolin, replication forks slow down and compromise forks convergence during S phase, thus increasing the risk of entering mitosis with under-replicated DNA. When mitosis ensues in the presence of under-replicated DNA, TRAIP promotes ubiquitylation of Mcm7 within stalled CMGs with long ubiquitin chains, followed by disassembly of the stalled replisomes by p97-Ufd1-Npl4 and DNA incisions in the leading strand templates. In this scenario, one of the chromatids is faithfully repaired through gap filling (1) while repair of the other one requires the joining of the two broken ends likely through the alt-NHEJ mechanisms of SSA and MMEJ. This chromatid, therefore, receives a deletion encompassing the under-replicated DNA and exhibits sister chromatid exchange (SCE) and potential templated insertions (2). (C) Disassembly of replisomes prior to ICL repair. Convergence of replication forks at both sites of the ICL triggers ubiquitylation of several CMG components by TRAIP. These ubiquitin chains are long and heterotypically linked and/or branched. Ubiquitylated CMGs are disassembled by p97, followed by fork reversal, nucleolytic incisions, ICL unhooking and DNA repair. One of the chromatids is repaired through translesion synthesis (1) while the other one is repaired through homologous recombination (2).