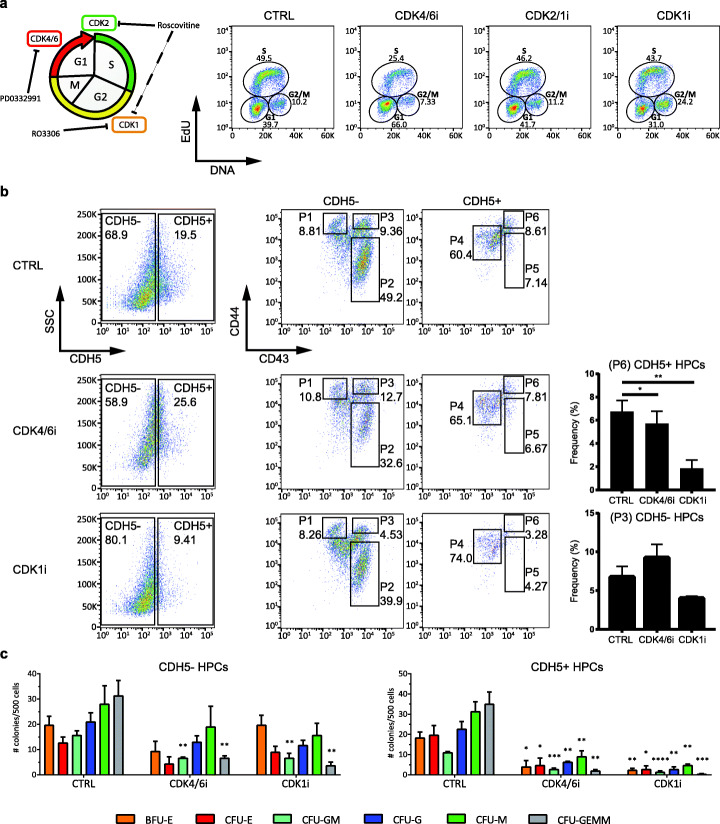
Fig. 6.
Functional effect of CDK inhibitors during EHT. a Schematic showing inhibitors used in the experiments (left panel) and their effect on cell cycle progression (right panel). Samples were treated for 48 h between EHT D3 and EHT D5 with either 0.1% DMSO (CTRL), 1 μM PD0332991 (CDK4/6i), 10 μM RO3306 (CDK1i), or 4 μM Roscovitine (CDK2/1i). At EHT D5, cells were either a processed for EdU staining and flow cytometry to monitor cell cycle profile or b stained for flow cytometry to monitor the frequency of distinct cell populations generated (P1, mesenchymal cells; P4, endothelial cells; P2 and P5, erythroid progenitors; P3 and P6, HPCs). Results are representative of 3 independent experiments. c At EHT D5, cells were washed to remove the inhibitors and two populations of HPCs (corresponding to P3 and P6 in b) were FACS-sorted and further cultured in a CFU assay to reveal their differentiation potential. Data are mean ± SEM of 3 independent experiments, with statistical significance compared to control as *P < 0.5, **P < 0.01, ***P < 0.001, and ****P < 0.0001 by one-way ANOVA. Degrees of freedom in b for CDH5− HPCs are DFn = 1.103, DFd = 2.205, F value 11.19, and for CDH5+ HPCs are DFn = 1.046, DFd = 2.092, F value 204.2. Degrees of freedom in c are DFn = 2, DFd = 6; F values for the distinct colonies in CDH5− HPCs are BFU-E = 2.483, CFU-E = 2.771, CFU-GM = 12.15, CFU-G = 3.261, CFU-M = 0.8633, and CFU-GEMM = 17.43; F values for the distinct colonies in CDH5+ HPCs are BFU-E = 12.51, CFU-E = 6.757, CFU-GM = 67.36, CFU-G = 21.71, CFU-M = 19.26, and CFU-GEMM = 30.63