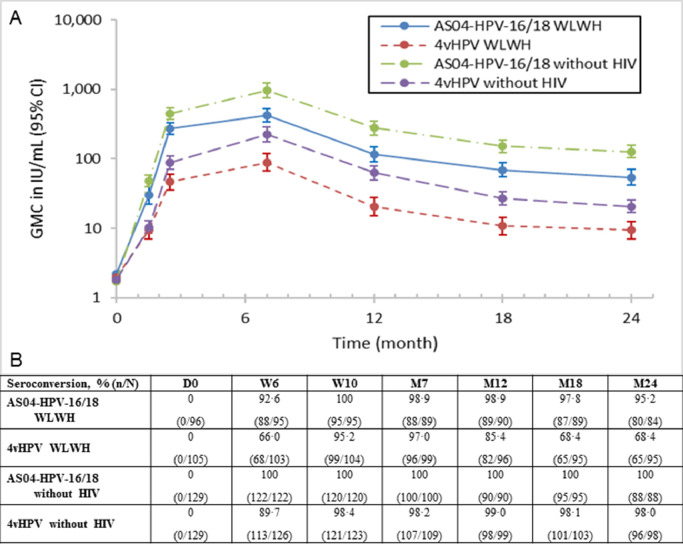
Fig. 4.
(A) Persistence of HPV-18 antibody titers (International Units per mL [IU/mL]) and (B) seroconversion rates in all subjects who received at least one dose of the vaccine (TVC). Seroconversion: percentage of subjects with an anti-HPV-18 VLP IgG antibody concentrations ≥3.2 IU/mL (by ELISA) ATP: according to protocol;D: Day; CI: confidence interval; ELISA: Enzyme-linked Immunosorbent Assay; GMC: geometric mean concentration; HIV: human immunodeficiency virus; HPV: human papillomavirus; IgG: immunoglobulin G; M: Month; n: number of subjects seroconverted; N: total number of subjects; VLP: virus-like particle; W: Week; WLWH: women living with HIV.