Fig. 1.
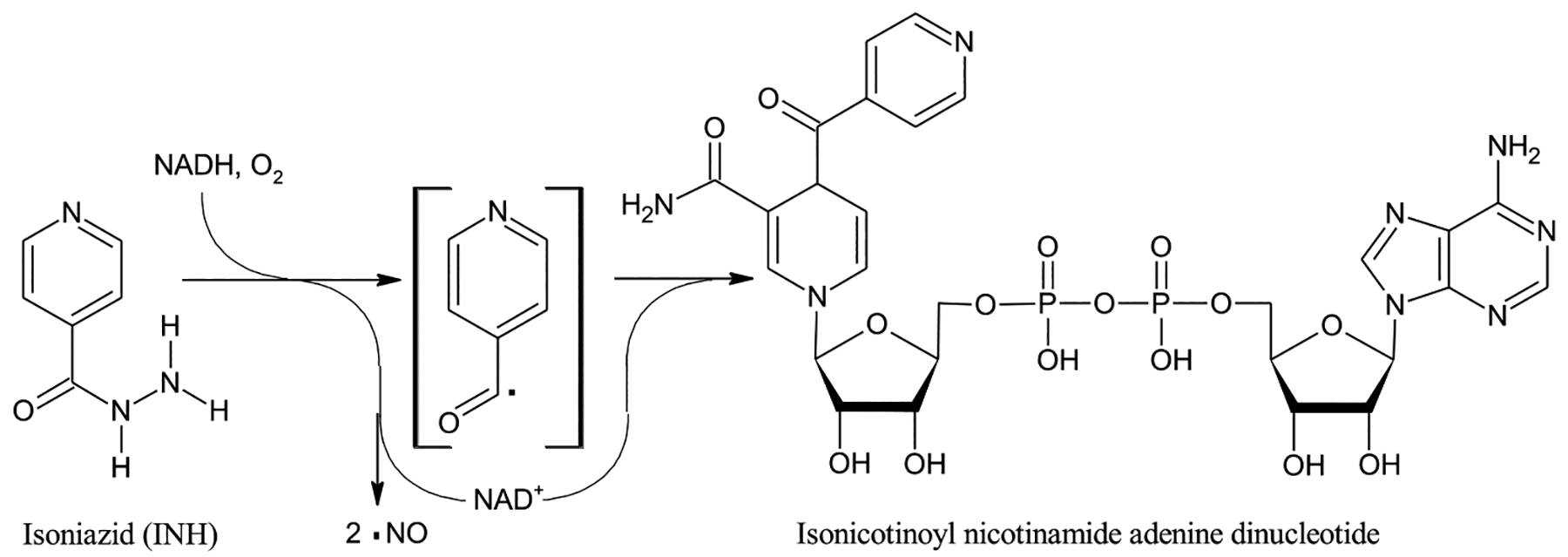
The structure of INH, also known as isonicotynylhydrazide or pyridine-4-carbohydrazide, along with a schematic version of the reaction of INH catalyzed by the catalase-peroxidase enzyme KatG based on findings of Singh et al. [9] and Timmons et al. [10] The free radical nature of the reaction and formation of NO is experimentally well-established [9,11] but not all intermediates may have been identified and the radical shown in brackets, while possibly a reaction intermediate, is for illustrative purposes only. Thus, no attempt is made to show a balanced reaction. The product isonicotinoyl nicotinamide adenine dinucleotide binds at the active site of the enzyme enoyl-acyl carrier protein reductase, stopping M. tuberculosis fatty acid synthesis, including the synthesis of the cell wall component myconic acids [10].
