Summary
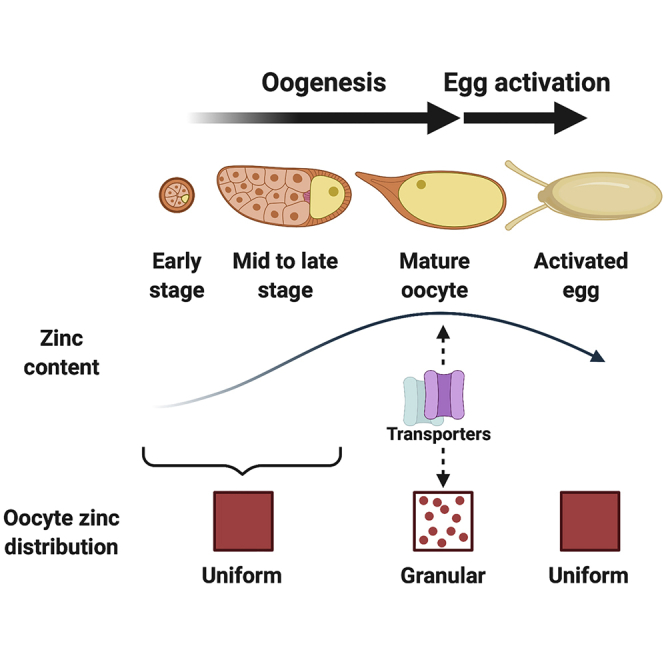
Temporal fluctuations in zinc concentration are essential signals, including during oogenesis and early embryogenesis. In mammals, zinc accumulation and release are required for oocyte maturation and egg activation, respectively. Here, we demonstrate that zinc flux occurs in Drosophila oocytes and activated eggs, and that zinc is required for female fertility. Our synchrotron-based X-ray fluorescence microscopy reveals zinc as the most abundant transition metal in Drosophila oocytes. Its levels increase during oocyte maturation, accompanied by the appearance of zinc-enriched intracellular granules in the oocyte, which depend on transporters. Subsequently, in egg activation, which mediates the transition from oocyte to embryo, oocyte zinc levels decrease significantly, as does the number of zinc-enriched granules. This pattern of zinc dynamics in Drosophila oocytes follows a similar trajectory to that in mammals, extending the parallels in female gamete processes between Drosophila and mammals and establishing Drosophila as a model for dissecting reproductive roles of zinc.
Subject Areas: Evolutionary Developmental Biology, Biotechnology, Embryology
Graphical Abstract
Highlights
-
•
Dietary zinc is required for Drosophila female fertility
-
•
Oocyte zinc levels increase over oogenesis and decrease during egg activation
-
•
Zinc is enriched in the oocyte and forms aggregated granules in mature oocytes
-
•
Transporters are required for zinc retention and zinc-enriched granules in oocytes
Evolutionary Developmental Biology; Biotechnology; Embryology
Introduction
Transition metals such as iron, copper, and zinc ions bound tightly within enzymatic sites play a variety of well-established structural and catalytic roles within cells (Calap-Quintana et al., 2017; Egli et al., 2003). Among them, zinc is an important inorganic regulator of processes including cell proliferation, carbohydrate metabolism, and immunity (reviewed in Frassinetti et al., 2006; Roohani et al., 2013). Zinc can persist in cells as hydrated ions, bound to small metabolites as weakly bound complex ions or bound tightly in the active sites of zinc-dependent enzymes. Recent studies indicate that fluctuations in the availability of Zn2+ ions occur either through ionic fluxes (Bernhardt et al., 2012) or through covalent binding to metalloregulatory proteins (Gilston et al., 2014). Thus, this element serves alongside calcium and phosphorus as an inorganic signal mediator that can activate biological switching processes. One example of zinc as an inorganic signal occurs during mammalian oocyte maturation and embryogenesis. Specifically, zinc accumulates in maturing mouse oocytes from the arrest at prophase of meiosis I to the arrest at metaphase of meiosis II (MII) (Kim et al., 2010), and this increase is dependent on maternally derived zinc transporters ZIP6 and ZIP10 (Kong et al., 2014). When mature mouse oocytes are fertilized, thus “activating” them to complete meiosis and begin embryogenesis, there is a rapid release of zinc from the oocyte (Kim et al., 2011). Such “zinc sparks” have been observed in eggs upon activation in the mouse (Kim et al., 2011; Que et al., 2015; Zhang et al., 2016), cow (Que et al., 2019), and human (Duncan et al., 2016). This zinc release is dependent on calcium oscillations during egg activation (Kim et al., 2011; Suzuki et al., 2010) and is mediated by dynamic movement and exocytosis of zinc-loaded vesicles (Que et al., 2015; Tokuhiro and Dean, 2018). The programmed loss of cellular zinc through zinc sparks is associated with resumption of the cell cycle (Kim et al., 2011) and modification of the zona pellucida to block polyspermy (Que et al., 2017; Tokuhiro and Dean, 2018). After fertilization, regulation of zinc is required for the first mitotic divisions during embryogenesis (Kong et al., 2015). In another model organism, C. elegans, zinc is also required for oogenesis, meiotic progression (Hester et al., 2017), and embryo viability (Mendoza et al., 2017).
Drosophila's speed of development and excellent genetics makes it a tractable model to probe mechanisms and macromolecules of relevance to more complex systems, and its biology includes many parallels to events that occur in Xenopus and mammals, including in reproduction (Avila et al., 2010; Barnard et al., 2004; Bernhardt et al., 2018; Cui et al., 2008; Horner et al., 2006; Hu and Wolfner, 2019; Knapp and Sun, 2017; Mochida and Hunt, 2007; Pepling and Spradling, 2001; Takeo et al., 2006, 2010; Zhang et al., 2018). As in other animals, Drosophila oocytes develop through a stepwise process of stages, 14 in this case, making it straightforward to examine zinc dynamics during oogenesis. Specifically, a cystoblast, the destined-to-differentiate daughter of a female germline stem cell, undergoes four mitotic divisions with incomplete cytokinesis to result in a 16-cell cyst. One of the 16 cells becomes the oocyte; her 15 sisters, the nurse cells, synthesize macromolecules, organelles, and other components that will be transferred into the oocyte as oogenesis progresses. Meiosis starts at early stage of oogenesis and arrests at prophase I at stage 5. This arrest lasts until stage 13 when meiosis progresses to metaphase I (MI) (von Stetina and Orr-Weaver, 2011). At later stages of oogenesis, nurse cells undergo apoptosis. The mature (stage 14) oocyte remains meiotically arrested at MI until ovulation (Figure 1A) (reviewed in Avilés-Pagán and Orr-Weaver, 2018; Bastock and St Johnston, 2008; McLaughlin and Bratu, 2015). Then, mechanical forces due to passage into the oviduct and/or swelling of the oocyte as it takes up oviductal fluid “activate” the egg (Heifetz et al., 2001, reviewed in Carlson, 2019; Horner and Wolfner, 2008a). As in mammals, egg activation in Drosophila involves a rise in internal free calcium levels (Kaneuchi et al., 2015; York-Andersen et al., 2015), progression through meiosis, and changes in the egg's transcriptome, proteome, and envelopes (reviewed in Horner and Wolfner, 2008a; Kashir et al., 2014; Krauchunas et al., 2013; Sartain and Wolfner, 2013; Swann and Lai, 2016). The process of egg activation can be largely, although not perfectly, mimicked in vitro by submerging isolated mature oocytes in a hypotonic buffer (Horner and Wolfner, 2008b; Page and Orr-Weaver, 1997).
Figure 1.
Dietary TPEN Impairs Female Drosophila Fertility
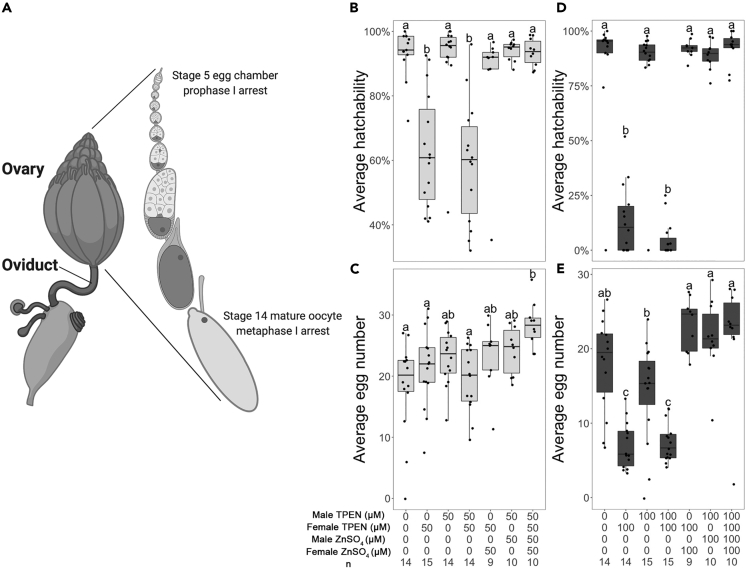
(A) Diagram of female Drosophila reproductive system, showing one of the pair of ovaries with enlarged view of the progressive stages in an ovariole. Meiotic stages during oogenesis are noted. Illustration created with Biorender.com.
(B–E) Three-day average egg hatchability (B and D) and egg number (C and E) from single-pair crosses of ORP2 females and males with either or both sexes raised in 50 μM (B and C) or 100 μM (D and E) TPEN only or TPEN + ZnSO4 rescue food. Male TPEN, concentration of dietary TPEN on which the males were raised and tested; female TPEN, concentration of TPEN added to food on which the females were raised and tested; male ZnSO4, concentration of ZnSO4 added to food on which the males were raised; female ZnSO4, concentration of ZnSO4 added to food on which the females were raised; n, sample size of each group; a, b, and c, significance groups (significant difference of mean with p < 0.05 between groups with different labels). Whiskers on the boxplots show the top and bottom quartiles.
Given the importance of zinc dynamics during mammalian and C. elegans oocyte maturation and egg activation, we asked if similar processes occur in Drosophila as well. Interestingly, in a previous proteomic study that looked at phospho-modulation of maternal proteins during the transition from egg to embryo, we noticed that Znt35C, the most highly expressed zinc transporter in the Drosophila ovary (Leader et al., 2017), undergoes phospho-state change during egg activation, suggesting that its activity might be modulated during egg activation (Zhang et al., 2018). Znt35C is one of the transporters that regulates the biogenesis of lysosome-related zinc storage granules in Drosophila malpighian tubules (Tejeda-Guzmán et al., 2018), excretory organs that are a site of very active zinc homeostasis regulation.
To further examine the role of zinc in Drosophila reproduction, we first tested whether zinc is important for fertility. We observed that females fed a zinc-deficient diet had impaired fertility. This prompted us to examine the dynamics of zinc in their germline. Using synchrotron-based X-ray fluorescence microscopy (XFM), we tracked the distribution and dynamics of zinc and other transition metals throughout Drosophila oogenesis and egg activation. We observed that zinc is the most abundant transition metal in Drosophila oocytes and eggs. Total intracellular zinc levels increase during oogenesis, accompanied by formation of zinc-enriched granules in the oocyte cytoplasm. The maintenance of these zinc granules in mature oocytes depends on transporters. Upon egg activation, there is a significant decrease in intracellular zinc levels and in the presence of zinc granules in wild-type oocytes. All these observations are reminiscent of zinc dynamics seen in mammalian oocyte maturation and egg activation.
Results
Dietary Zinc Deficiency Reduces Female Drosophila Fertility
Given the importance of zinc in the mammalian and C. elegans oocyte, we began by testing whether zinc is essential for female fertility in Drosophila. We followed protocols analogous to those reported for C. elegans (Hester et al., 2017), where addition of the zinc chelator, N,N,N′,N'-tetrakis(2-pyridylmethyl)-1,2-ethylenediamine (TPEN) to the food at a concentration of 50 μM significantly impairs C. elegans fertility. We raised Oregon-R-P2 (ORP2; wild-type) (Allis et al., 1977) flies on food containing 50 or 100 μM TPEN and assessed their fecundity and egg hatchability (percent of eggs that are able to hatch) on food containing TPEN. We tested single-pair crosses, in which one or both partners had been raised on food containing TPEN and compared them with control crosses, in which both partners had been raised on control food. Males raised on 50 or 100 μM TPEN food were not impaired in siring offspring; their mates laid normal numbers of eggs that had normal hatchability (Figures 1B–1E). Female flies raised on either 50 or 100 μM TPEN-treated food exhibited reduced egg hatchability; the effect was more severe at a higher dosage of TPEN, regardless of whether their mates came from control or TPEN food (Figures 1B and 1D). It was observed that 50 μM TPEN-treated females displayed reduced egg hatchability only beginning on day 2 postmating (Figure S1A). To test if this was the result of residual zinc from their parents, who had been reared on untreated food and transferred to TPEN-treated food after eclosion, we repeated the assay using female offspring of TPEN-treated flies that themselves had been reared on TPEN-treated food. We observed the same trend (Figure S1B), suggesting that addition of 50 μM TPEN to food leads to a delayed reduction in egg hatchability. Females raised on 50 μM TPEN laid normal numbers of eggs (Figure 1C), whereas females raised on 100 μM TPEN produced significantly fewer eggs (Figure 1E). All these adverse effects on female fertility were rescued by supplementation with ZnSO4 at equimolar concentrations to the TPEN in the food (Figures 1B–1E), confirming the requirement of zinc for female fertility. Thus, dietary zinc is required for female Drosophila fertility, but does not appear to affect male fertility. Moreover, zinc insufficiency impacts both the quality and quantity of oocytes.
As TPEN also has a high affinity for copper (Percival and Layden-Patrice, 1992), we attempted to test the effects of two copper chelators, neocuproine and ammonium tetrathiomolybdate (TM), on Drosophila female fertility. Unfortunately, at all concentrations tested (25, 50, and 100 μM, based on comparative concentrations of TPEN), presence of either chelator in the medium was either toxic or semitoxic to the flies (neocuprione) or slowed the flies' development by > 3-fold (TM), indicating a negative effect on fitness. Thus, although the surviving females had lower egg-laying ability and hatchability than normal (reduction of 13%, 73% for 25 μM neocuprione; 41%, 88% for 50 μM neocuprione; 63%, 91% for 25 μM TM; and 52%, 95% for 50 μM TM, respectively), this cannot be considered direct effects of either chelator on the female germline; it is likely a consequence of the toxicity/negative fitness effects of the chelator to the fly as a whole.
Zinc Is the Most Abundant Transition Metal Measured in Drosophila Oocytes and Activated Eggs
Given the importance of zinc in mammalian and C. elegans gamete biology and the effect of dietary zinc deficiency in female Drosophila fertility, we measured and visualized the subcellular distribution of total zinc as well as other transition elements in oocytes from ORP2 females using synchrotron-based XFM. We examined four groups of egg chambers and eggs throughout development: early-stage oogenesis (stages 1–8), mid/late-stage oogenesis (stages 9–13), mature oocytes (stage 14), and activated (laid, unfertilized) eggs (Figures 2A and 2B). XFM data provide the content measurements of a sample through detection of element-specific X-ray emission spectra. The total amount of each element (in fmol) was calculated from data integrated along the z axis of the scans of each sample. Element distributions were represented as two-dimensional projection images with total element content measurements. We analyzed the element contents of the egg chambers (including nurse cells and oocytes) as well as the oocytes alone (for stages with clearly observable oocytes). The oocyte can be distinguished as the most posterior cell within the egg chamber. As nurse cells dump their cytoplasm into the oocyte during late oogenesis (reviewed in Cavaliere et al., 1998), the element contents of mature oocytes and activated eggs should include those from both nurse cells and oocytes from earlier stages of oogenesis. We also attempted to quantify element concentration in scanned samples. As the volumes of egg chambers were difficult to measure due to their irregular shape, we used total element amounts in the region of interest (ROI) divided by the area of ROI as an approximate of element concentration.
Figure 2.
Zinc Level and Distribution Changes during Oocyte Maturation and Egg Activation in Wild-Type Drosophila
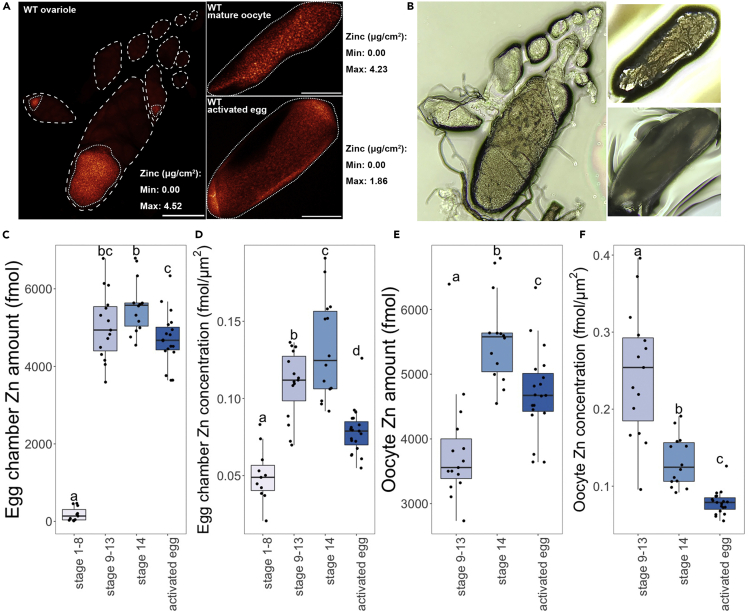
(A) Representative XFM images showing zinc distribution in egg chambers at different stages of oogenesis and egg activation. Left, an ovariole containing immature egg chambers up to stage 10; upper right, a stage 14 mature oocyte; lower right, an activated but unfertilized egg. Dashed lines delineate the outline of egg chambers. Dotted lines delineate the outline of oocytes in stage 9–13 egg chambers, mature oocytes, and activated eggs.
(B) Bright-field image of samples scanned in (A). Due to dehydration during sample preparation, morphology of these egg chambers does not completely reflect their in vivo state. All scale bars, 100 μM.
(C–F) Total zinc content (C and E) and zinc concentration (D and F) of zinc in egg chambers (C and D) and oocytes (E and F). Stage 1–8 oocytes n = 11, stage 9–13 oocytes n = 15, stage 14 oocytes n = 14, activated eggs n = 19. a, b, c, and d, significance groups (significant difference of mean with p < 0.05 between groups with different labels). Whiskers on the boxplots show the top and bottom quartiles.
Consistent with their important physiological roles, zinc, iron, and copper were the most abundant transition metals measured across all stages of oogenesis and after egg activation. Among these three, zinc was present in the highest molar amount with 5,543 ± 684 fmol in mature oocytes, compared with 2,188 ± 344 fmol of iron and 198 ± 49 fmol of copper in mature oocytes (mean ± SEM, Figure S2A). This is analogous to the relative amounts of transition metals in mammalian oocytes and embryos (Kim et al., 2010).
Fluxes in Total Zinc Occur during Oocyte Maturation and Egg Activation
XFM showed increases in iron, copper, and zinc content as egg chambers transitioned from early to mid/late oogenesis. Before about stage 9, there are on average 6.24 ± 5.71 fmol of copper, 112 ± 95 fmol of iron, and 186 ± 178 fmol of zinc in each egg chamber. After stage 9, each egg chamber contained on average 156 ± 36 fmol of copper, 2,174 ± 307 fmol of iron, and 5,045 ± 879 fmol of zinc (mean ± SEM, Figure S2A). Interestingly, as the amount of these transition metals increased within egg chambers in mid-late oogenesis, copper, iron, and zinc became more concentrated in the oocyte, relative to the associated nurse cells. In stage 9–13 egg chambers, 71.1% copper, 51.5% iron, and 75.9% zinc were enriched in the oocyte, on average (Figures 2A and S3B).
From mid/late oogenesis stages to stage 14 and egg activation in vivo, both the amount and area concentration of iron and copper remained constant with no statistical differences in either egg chambers or oocytes alone (Figure S2). However, both the zinc content (fmol) and the zinc concentration (fmol/μm2) underwent dynamic changes (Figures 2C–2F and S2). When considering the entire egg chamber, zinc concentration significantly increased from stage 9–13 to stage 14 (Figure 2D). Zinc content and concentration significantly dropped from stage 14 oocytes to eggs activated in vivo (Figures 2C and 2D). When looking at the oocyte alone, zinc amount significantly increased from stage 9–13 to stage 14 and also significantly decreased from stage 14 to activated eggs (Figure 2E). Interestingly, oocyte zinc concentration kept dropping significantly as the oocytes progressed from stage 9–13 to stage 14 and from stage 14 to activated eggs in vivo (Figure 2F).
To rule out the possibility that the zinc level changes we observed were due to fluctuations of metal levels in general, we reanalyzed our data by normalizing the amount of zinc to that of iron. We saw the same trend: egg chamber zinc concentration rose significantly during oogenesis and dropped significantly during egg activation, and oocyte zinc concentration dropped significantly from stage 9–13 to stage 14 and from stage 14 to activated eggs (Figure S4).
Taken together, these data indicate that zinc content keeps increasing in the egg chamber over oogenesis but decreases after egg activation. Within the egg chamber, zinc becomes more and more enriched in the oocyte in the egg chamber over oogenesis before a significant decrease during egg activation. The reduced concentration from stage 9–13 to stage 14 mature oocyte (Figure 2F) is possibly due to nurse cell dumping, in which the zinc-enriched oocyte cytoplasm is mixed with the nurse cell cytoplasm containing less zinc.
Zinc-Enriched Granules Form during Oocyte Maturation and Diminish during Egg Activation
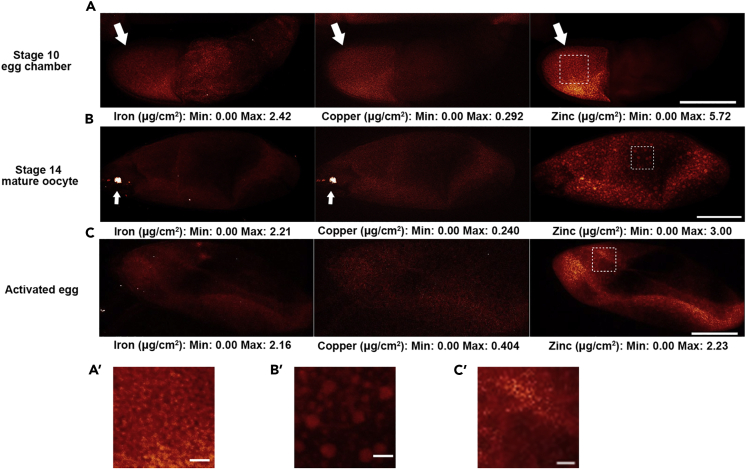
Although copper and iron displayed generally uniform distributions within the oocyte (Figures 3A and 3B), the zinc distribution became punctate over development (Figures 3A, 3A′, S3A, and S3B). In stage 14 mature oocytes, zinc was seen in distinct granules (Figures 3B, 3B′, S3C, and S3D). However, during egg activation, as intracellular zinc levels decreased, these zinc granules also decreased in number (Figures 3C, 3C′, S3E, and S3F). These data suggest that these granules may store zinc during oocyte maturation before the loss observed following egg activation. To further test for the existence of a granular or organellar basis for the distribution of zinc, we used a centrifugation protocol. Such treatment of Drosophila embryos is known to stratify their subcellular components (Tran and Welte, 2010). In activated eggs whose cytoplasm was stratified in this manner, the majority of iron, copper, and zinc became localized at the posterior end of the centrifuged eggs (Figure S5). This is where heavier organelles pellet, consistent with zinc being in or associated with organellar granules in the eggs. We could not examine the results for mature oocytes because they all collapsed during centrifugation, likely due to their fragile eggshells (Heifetz et al., 2001, reviewed in Horner and Wolfner, 2008a).
Figure 3.
Distribution of Iron, Copper, and Zinc during Oocyte Maturation and Egg Activation in Wild-Type Drosophila
(A–C) Representative XFM images showing iron, copper, and zinc distribution in (A) an egg chamber around stage 10. Within an egg chamber, iron, copper, and zinc are all more concentrated in the oocyte (arrows); (B) a mature oocyte (stage 14). Zinc displays a more granulated distribution compared with iron and copper. Iron and copper display an even distribution. A granule of highly concentrated iron and copper (arrows) was observed at the anterior end of 8 of 14 of the wild-type mature oocytes, at approximately the location of the future micropyle, and egg anterior cytoplasm (see additional examples in Figure S9). The nature of this granule is unknown; (C) an activated but unfertilized egg. Zinc displays an even distribution. All samples are from ORP2 wild-type females. Scale bars, 100 μm. (A′–C′) Enlarged view of zinc distribution in (A–C). Dashed squares indicate enlarged regions. Scale bars, 10 μm.
Transporters White (W) and Znt35C are important in the biogenesis of zinc storage granules in Drosophila malpighian tubules, an excretory tissue that is the Drosophila counterpart of the mammalian kidney (Tejeda-Guzmán et al., 2018). In particular, Znt35C (the ortholog of mammalian ZNT2/ZNT3/ZNT8), which localizes to the membranes of zinc storage granules in malpighian tubules (Tejeda-Guzmán et al., 2018), is the most highly expressed zinc transporter in the Drosophila ovary (Leader et al., 2017) and is regulated by phosphorylation during egg activation (Zhang et al., 2018). We generated a znt35C1 null mutation using CRISPR/Cas9; for technical reasons (Figure S10) our mutant strain also carried a mutation in the w gene. A previously published, but unavailable, znt35C null mutant had been reported to be viable unless exposed to excessive zinc (Yepiskoposyan et al., 2006); our znt35C1 mutant was similarly viable on normal food. Thus, we were able to isolate oocytes at all developmental stages from homozygous znt35C1 females and subject them to XFM analysis (Tejeda-Guzmán et al., 2018).
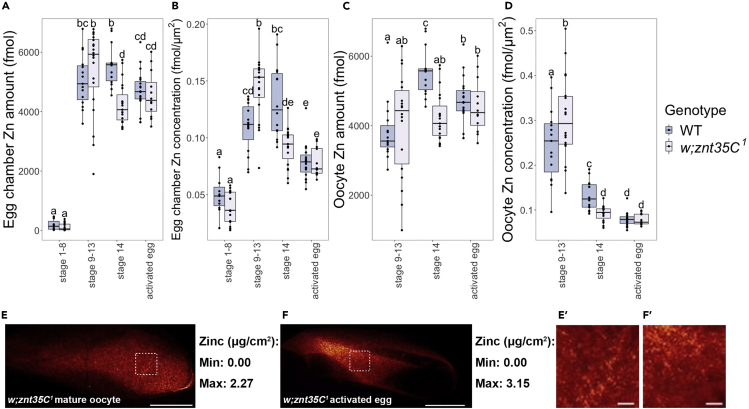
Our XFM data showed that zinc is present during oogenesis in w; znt35C1 mutants and was still the transition metal with the highest amount and concentration in egg chambers and oocytes (Figures S6A–S6D). As in the wild-type animals, iron, copper, and zinc were enriched in the oocyte relative to the whole egg chamber in the mutants (Figures S6E and S6E′). w; znt35C1 egg chambers displayed accumulation of zinc from stage 1–8 to stage 9–13 of oogenesis similar to wild-type (Figures 4A and 4B). However, w; znt35C1 oocytes contained more zinc than wild-type oocytes in stage 9–13 when looking at the egg chamber or the oocyte alone. This difference was statistically significant with regard to zinc concentration in egg chambers (Figure 4B) and oocytes (Figure 4D). In contrast, wild-type stage 14 mature oocytes displayed significantly higher zinc content and concentration than stage 14 oocytes of w; znt35C1 (Figures 4A and 4B). When looking at the oocyte alone, unlike that in wild-type oocytes, zinc amounts in w; znt35C1 oocytes remained constant from stage 9–13 to stage 14 and from stage 14 to activated eggs (Figure 4C). These trends remained the same when zinc content was normalized to iron content (Figure S4). Zinc granules were also largely absent from w; znt35C1 mature oocytes (Figures 4E, 4E′, S6F, and S6F′) or activated eggs (Figures 4F, 4F′, S6G, and S6G′). However, w; znt35C1 females did not display defects in egg production or hatchability (Figure S7), suggesting that the accumulation of zinc in mature oocytes is not solely (or non-redundantly) necessary for Drosophila fertility under zinc-sufficient conditions.
Figure 4.
Zinc Distribution and Quantification Over Oocyte Maturation and Egg Activation in w; znt35C1Drosophila
(A–D) The total zinc content (A and C) and zinc concentration (B and D) in each egg chamber (A and B) and oocyte (C and D) of w; znt35C1 compared with ORP2 wild-type. WT: ORP2 wild-type. KO: w; znt35C1 null mutant. Stage 1–8 oocytes: WT n = 11, KO n = 15; stage 9–13 oocytes: WT n = 15, KO n = 20; stage 14 oocytes: WT n = 14, KO n = 19; activated eggs: WT n = 19, KO n = 12. a, b, c, d, and e: significance groups (significant difference of mean with p < 0.05 between groups with different labels). Whiskers on the boxplots show the top and bottom quartiles. (E and F) Representative XFM images showing zinc distribution in (E) a mature oocyte at stage 14 and (F) an activated but unfertilized egg from w; znt35C1 mutant females. Scale bars, 100 μm. (E′ and F′) Enlarged view of (E and F). Dashed squares indicate enlarged regions. Scale bars, 10 μm.
Taken together, our data showed that in w; znt35C1 mutants, zinc accumulates more in egg chambers and enriches more in oocytes compared with wild-type before stage 14. At stage 14, w; znt35C1 oocytes fail to retain zinc as well as wild-type does, concurrent with the absence of zinc-enriched granules. A subsequent zinc decrease during egg activation, observed in wild-type, is not observed in w; znt35C1 mutants. Our data suggest that one or both of these transporters have roles in transporting zinc out of egg chambers before oocyte maturation and/or roles in retaining zinc in mature oocytes by maintaining zinc-enriched granules; future studies will tease apart the roles of these two transporters in zinc homeostasis.
Discussion
Zinc is essential for life and necessary for reproductive functions and fertility in C. elegans and several mammalian species. However, the requirement for zinc in fertility and its levels and distributions in oocytes and after oogenesis were unknown for any arthropod, including the major model system, Drosophila. Here, we determined that dietary zinc is essential for Drosophila female fertility. This prompted us to use XFM to examine zinc distribution during oogenesis and egg activation. We found that zinc is the most abundant transition metal in Drosophila oocytes at all stages. Zinc levels increase during oogenesis, and within the egg chamber, zinc becomes concentrated in the oocyte. Mature oocytes have high levels of zinc in aggregates or granules. Egg activation coincides with loss of both zinc and granules from the oocyte. As discussed later in the article, the loading of zinc into oocyte granules and the loss of zinc upon egg activation are conserved between fly and mammalian oocyte biology.
Zinc Is Required for Female Fertility in Drosophila
Dietary zinc is necessary for female fertility in Drosophila, impacting both the quality and quantity of eggs produced, suggesting the fly as a possible model for studying zinc-related subfertility. In mammalian females, zinc also plays multiple roles in oocytes across various stages. Dietary zinc depletion leads to premature germinal vesicle breakdown, spindle defects during oocyte maturation, and blocked ovulation and epigenetic programming alterations (Tian and Diaz, 2013, 2012). After fertilization, zinc deficiency further perturbs chromatin structure, reduces global transcription, and disrupts placental development during early embryogenesis (Kong et al., 2015; Tian et al., 2014). In C. elegans hermaphrodites, dietary zinc deficiency results in impaired oogenesis and chromosome dynamics during meiosis in the germline, which reduces brood size and embryo viability (Hester et al., 2017; Mendoza et al., 2017). Interestingly, we did not see impairment of male fertility upon zinc depletion in Drosophila males, even though in mammals zinc deficiency is associated with sperm chromatin instability and thus male infertility (Caldamone et al., 1979; Kvist et al., 1987). This observation is analogous to C. elegans, in which oocytes are more sensitive to zinc deprivation than sperm (Mendoza et al., 2017). It is still possible that the levels of TPEN we tested in Drosophila, although sufficient to impair female fertility, were too low to affect spermatogenesis. In addition, dietary zinc deficiency could also lead to impaired female fertility in a systematic and indirect way instead of through a direct impact on oogenesis. These possibilities are subjects for future studies.
Transporters Are Required for the Maintenance of Zinc Storage Granules in Drosophila Oocytes
During Drosophila oocyte maturation, the presence of zinc-enriched granules is maintained by zinc transporter Znt35C and/or the ABC transporter W; future studies are required to distinguish their relative contributions. Drosophila Znt35C and W are also involved in the biogenesis of lysosome-related zinc granules, which function as the major zinc reservoir in principal malpighian tubule epithelial cells (Tejeda-Guzmán et al., 2018). It is thus tempting to speculate that similar mechanisms are used in the Drosophila oocyte to store its high levels of zinc during maturation in preparation for zinc release upon egg activation. Consistent with this interpretation, we observed that w; znt35C1 null mutant females fail to maintain high levels of zinc or zinc granules in their mature oocytes and consequently show no decrease in zinc levels upon egg activation.
In mammalian egg activation, zinc is released from zinc-loaded vesicles undergoing exocytosis after fertilization (Que et al., 2015). Given that zinc granules in wild-type Drosophila mature oocytes are largely absent after egg activation, they may serve similar purposes in packaging zinc for traffic out of oocytes during egg activation. It will be intriguing in this context to examine the role of the mammalian orthologs of W (ABCG2) and of Znt35C (ZNT2/ZNT3/ZNT8; members of the SLC30 [ZnT] family of membrane proteins, which transport zinc out of the cytosol, some of which are localized in the plasma membrane and others in intracellular compartments; reviewed in Schweigel-Röntgen, 2014) in organizing and maintaining zinc levels in mammalian oocytes and in the mechanics of zinc release upon fertilization.
Zinc Dynamics during Oocyte Maturation and Egg Activation in Drosophila and Mammals
In multiple mammalian species, zinc levels are high in oocytes but drop dramatically during egg activation via a series of exocytotic events termed zinc sparks (Duncan et al., 2016; Kim et al., 2011; Que et al., 2019; Zhang et al., 2016). In mice, the zinc spark occurs coordinately with and is dependent on calcium oscillations during egg activation (Kim et al., 2011) and plays multiple roles in downstream events of egg activation including resumption of cell cycle (Kim et al., 2011) and modification of the zona pellucida (Que et al., 2017). Our observation of a decrease in zinc levels in Drosophila oocytes during egg activation parallels what is seen in mammals at this time, suggesting an overall analogy in phenomena, and the potential for Drosophila to serve as a model for dissecting zinc flux mechanisms. In mouse, mature oocytes arrest at MII stage of meiosis when intracellular zinc level peaks. The zinc spark is required for release of MII arrest and completion of meiosis (Kim et al., 2011). Drosophila mature oocytes arrest at MI of meiosis. Meiosis also completes after egg activation. It is possible that more complex zinc fluxes occur as meiosis progresses during egg activation (e.g., increase before MII and decrease afterward, parallel to that in mouse). However, as we only examined oocyte samples before and after egg activation here, the presence of such dynamics is a subject of future studies.
Interestingly, we did not see a decrease in zinc granules following in vitro activation (Figures S8A and S8A′); similarly, we only saw the decrease in zinc levels during egg activation in vivo but not after in vitro egg activation (Figure S8B). There are two possible explanations for this difference between eggs activated in vivo and in vitro. It is possible that zinc release and reduction of zinc granules occurs after the 30-min window used for the in vitro experiments. Alternatively, in vitro activation is known not to be completely physiological (Horner and Wolfner, 2008b; Page and Orr-Weaver, 1997), and it may thus fail to modulate zinc levels appropriately. However, the failure of zinc changes in vitro to match those seen in vivo made it impossible for us to directly observe zinc release real-time in Drosophila egg activation with fluorescent zinc markers.
Our findings that zinc accumulates during Drosophila oogenesis, becomes concentrated in granules in the mature oocyte, and eventually decreases after egg activation suggests possible conservation of zinc dynamics from Drosophila to those in C. elegans and mammals. Similar mechanisms (e.g., homologous zinc transporters) may be involved in zinc homeostasis regulation during oocyte maturation and egg activation. This strongly motivates the studies of these parallel processes from Drosophila to C. elegans and mammals.
Limitations of the Study
The irregular shape of the oocytes, particularly after the required dehydration, prevented precise calculation of the concentration of elements measured by dividing the total elemental content by the volume of egg chambers or oocytes. We divided the total elemental content by the area of ROI as an approximation. The relative data are correct, but the absolute number may have an incalculable slight margin of error. In the future, we may be able to approximate oocyte volumes by expressing fluorescent plasma membrane markers throughout oogenesis, acquiring z stack images of egg chambers with confocal imaging, reconstructing the 3D model of egg chambers, and measuring its volume. Using this approach, we could better assess an average egg chamber volume at different stages of oogenesis for more precision in element concentration.
As XFM only provides 2D images stacked along the z axis of scanned samples, we were unable to quantify the number and size changes of zinc-enriched granules or determine their intracellular localization through oogenesis and egg activation. Such experiments may reveal the molecular nature of these granules and will be the subject of future studies.
To investigate the role of Znt35C mutant, we generated a znt35C1 null mutant strain using CRISPR/Cas9. However, due to technical limitations, our mutant strain was in the w- background. Mutations in w impact the zinc physiology of flies, including their zinc storage ability (Afshar et al., 2013; Tejeda-Guzmán et al., 2018). Thus, we were unable to determine if the reduction in zinc levels and zinc granule numbers were results from mutation of w, znt35C, or both. Future analysis of samples from w+; znt35C1 will be required to clarify this.
Resource Availability
Lead Contact
M.F. Wolfner (mariana.wolfner@cornell.edu) is the lead contact for this paper.
Materials Availability
The w; znt35C1 mutant strain is available upon request from M. Wolfner.
Data and Code Availability
The XFM raw data are available at https://drive.google.com/drive/folders/1O9wDQbawoZ2Ytp9LwcLB2hCFDFwATuXe?usp=sharing; R codes used for analysis and plotting are available at https://github.com/WolfnerLab/zinc_reproduction.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. We thank Cornell's graduate school travel award (to Q.H.) and George P. Hess travel award (to Q.H.); National Institutes of Health grants R01-GM115848 (to T.K.W. and T.V.O.), R01-GM038784 (to T.V.O.), and R21-HD088744 (to M.F.W.); Postdoctoral Individual National Research Service Award F32-GM115052 (to A.B.N.); and Stephen H. Weiss Presidential Fellowship (to M.F.W.) and Barbara Payne Memorial Funds (to M.F.W.) for funding this study. We thank Dr. Robert A. Holmgren for rearing flies for some of the experiments. We thank Dr. Yasir Ahmed-Braimah for suggesting the egg centrifugation experiment and Drs. J. Liu, =C. Han, S. Garwin, and three anonymous reviewers for helpful comments on the manuscript. We thank Adriana N. Vélez-Avilés for assistance with znt35C1 mutant strain screening and Lauryn A. Worley for assistance with the TPEN fertility assays, and the Society for Developmental Biology's “Choose Development!” fellows' program (NSF grants IOS-1239422 and REU DBI-1156528) and Cornell Molecular Biology and Genetics department's Research Experience for Undergraduates (NSF grant REU DBI-1659534) program for supporting them. We thank Cornell's Statistical Consulting Unit for assistance with data analysis. The diagrams for the Graphical Abstract were created using Biorender.
Author Contributions
Conceptualization, Q.H., F.E.D., and M.F.W; Investigation, Q.H., F.E.D., A.B.N., and O.A.A.; Data Analysis, Q.H., O.A.A., F.E.D., M.F.W., T.K.W., and T.V.O.; Writing, Q.H.; Editing, Q.H., F.E.D., A.B.N., O.A.A., T.K.W., T.V.O., and M.F.W.; Funding Acquisition, T.K.W., T.V.O., and M.F.W.
Declaration of Interests
The authors declare no conflict of interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101275.
Contributor Information
Teresa K. Woodruff, Email: tkw@northwestern.edu.
Thomas V. O'Halloran, Email: t-ohalloran@northwestern.edu.
Mariana F. Wolfner, Email: mariana.wolfner@cornell.edu.
Supplemental Information
References
- Afshar N., Argunhan B., Bettedi L., Szular J., Missirlis F. A recessive X-linked mutation causes a threefold reduction of total body zinc accumulation in Drosophila melanogaster laboratory strains. FEBS Open Biol. 2013;3:302–304. doi: 10.1016/j.fob.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis C.D., Waring G.L., Mahowald A.P. Mass isolation of pole cells from Drosophila melanogaster. Dev. Biol. 1977;56:372–381. doi: 10.1016/0012-1606(77)90277-9. [DOI] [PubMed] [Google Scholar]
- Avila F.W., Sirot L.K., LaFlamme B.A., Rubinstein C.D., Wolfner M.F. Insect seminal fluid proteins: identification and function. Annu. Rev. Entomol. 2010;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilés-Pagán E.E., Orr-Weaver T.L. Activating embryonic development in Drosophila. Semin. Cell Dev. Biol. 2018;84:100–110. doi: 10.1016/j.semcdb.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard D.C., Ryan K., Manley J.L., Richter J.D. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell. 2004;119:641–651. doi: 10.1016/j.cell.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Bastock R., St Johnston D. Drosophila oogenesis. Curr. Biol. 2008;18:R1082–R1087. doi: 10.1016/j.cub.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Bernhardt M.L., Kong B.Y., Kim A.M., O’Halloran T.V., Woodruff T.K. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol. Reprod. 2012;86:111–114. doi: 10.1095/biolreprod.111.097253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt M.L., Stein P., Carvacho I., Krapp C., Ardestani G., Mehregan A., Umbach D.M., Bartolomei M.S., Fissore R.A., Williams C.J. TRPM7 and Ca V3.2 channels mediate Ca 2+influx required for egg activation at fertilization. Proc. Natl. Acad. Sci. U S A. 2018;115:E10370–E10378. doi: 10.1073/pnas.1810422115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calap-Quintana P., González-Fernández J., Sebastiá-Ortega N., Llorens V.J., Moltó D.M. Drosophila melanogaster models of metal-related human diseases and metal toxicity. Int. J. Mol. Sci. 2017;18:1456. doi: 10.3390/ijms18071456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldamone A.A., Freytag M.K., Cockett A.T.K., Cockett T.K. Seminal zinc and male infertility. Urology. 1979;13:280–281. doi: 10.1016/0090-4295(79)90421-7. [DOI] [PubMed] [Google Scholar]
- Carlson A.E. Mechanical stimulation activates Drosophila eggs via Trpm channels. Proc. Natl. Acad. Sci. U S A. 2019;116:18757–18758. doi: 10.1073/pnas.1913150116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere V., Taddei C., Gargiulo G. Apoptosis of nurse cells at the late stages of oogenesis of Drosophila melanogaster. Dev. Genes Evol. 1998;208:106–112. doi: 10.1007/s004270050160. [DOI] [PubMed] [Google Scholar]
- Cui J., Sackton K.L., Horner V.L., Kumar K.E., Wolfner M.F. Wispy, the Drosophila homolog of GLD-2, is required during oogenesis and egg activation. Genetics. 2008;178:2017–2029. doi: 10.1534/genetics.107.084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan F.E., Que E.L., Zhang N., Feinberg E.C., O’Halloran T.V., Woodruff T.K. The zinc spark is an inorganic signature of human egg activation. Sci. Rep. 2016;6:24737. doi: 10.1038/srep24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli D., Selvaraj A., Yepiskoposyan H., Zhang B., Hafen E., Georgiev O., Schaffner W. Knockout of ‘metal-responsive transcription factor’ MTF-1 in Drosophila by homologous recombination reveals its central role in heavy metal homeostasis. EMBO J. 2003;22:100–108. doi: 10.1093/emboj/cdg012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frassinetti S., Bronzetti G., Caltavuturo L., Cini M., Croce C. Della. The role of zinc in life: a review. J. Environ. Pathol. Toxicol. Oncol. 2006;25:597–610. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i3.40. [DOI] [PubMed] [Google Scholar]
- Gilston B.A., Wang S., Marcus M.D., Canalizo-Hernandez M.A., Swindell E.P., Xue Y., Mondragon A., O’Halloran T.V. Structural and mechanistic basis of zinc regulation across the E. coli Zur regulon. Plos Biol. 2014;12:e1001987. doi: 10.1371/journal.pbio.1001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y., Yu J., Wolfner M.F. Ovulation triggers activation of Drosophila oocytes. Dev. Biol. 2001;234:416–424. doi: 10.1006/dbio.2001.0246. [DOI] [PubMed] [Google Scholar]
- Hester J., Hanna-Rose W., Diaz F. Zinc deficiency reduces fertility in C. elegans hermaphrodites and disrupts oogenesis and meiotic progression. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017;191:203–209. doi: 10.1016/j.cbpc.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner V.L., Czank A., Jang J.K., Singh N., Williams B.C., Puro J., Kubli E., Hanes S.D., Mckim K.S., Wolfner M.F., Goldberg M.L. The Drosophila calcipressin sarah is required for several aspects of egg activation. Med. Eng. Phys. 2006;16:1441–1446. doi: 10.1016/j.cub.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Horner V.L., Wolfner M.F. Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev. Dyn. 2008;237:527–544. doi: 10.1002/dvdy.21454. [DOI] [PubMed] [Google Scholar]
- Horner V.L., Wolfner M.F. Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila oocytes in vitro in a calcium-dependent manner. Dev. Biol. 2008;316:100–109. doi: 10.1016/j.ydbio.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Wolfner M.F. The Drosophila Trpm channel mediates calcium influx during egg activation. Proc. Natl. Acad. Sci. U S A. 2019;116:18994–19000. doi: 10.1073/pnas.1906967116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneuchi T., Sartain C.V., Takeo S., Horner V.L., Buehner N.A., Aigaki T., Wolfner M.F. Calcium waves occur as Drosophila oocytes activate. Proc. Natl. Acad. Sci. U S A. 2015;112:791–796. doi: 10.1073/pnas.1420589112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashir J., Nomikos M., Lai F.A., Swann K. Sperm-induced Ca2+ release during egg activation in mammals. Biochem. Biophys. Res. Commun. 2014;450:1204–1211. doi: 10.1016/j.bbrc.2014.04.078. [DOI] [PubMed] [Google Scholar]
- Kim A.M., Bernhardt M.L., Kong B.Y., Ahn R.W., Vogt S., Woodruff T.K., Halloran T.V.O. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 2011;6:716–723. doi: 10.1021/cb200084y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A.M., Vogt S., O’Halloran T.V., Woodruff T.K. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 2010;6:674–681. doi: 10.1038/nchembio.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp E., Sun J. Steroid signaling in mature follicles is important for Drosophila ovulation. Proc. Natl. Acad. Sci. U S A. 2017;114:699–704. doi: 10.1073/pnas.1614383114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B.Y., Duncan F.E., Que E.L., Kim A.M., O’Halloran T.V., Woodruff T.K. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol. Hum. Reprod. 2014;20:1077–1089. doi: 10.1093/molehr/gau066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B.Y., Duncan F.E., Que E.L., Xu Y., Vogt S., O’Halloran T.V., Woodruff T.K. The inorganic anatomy of the mammalian preimplantation embryo and the requirement of zinc during the first mitotic divisions. Dev. Dyn. 2015;244:935–947. doi: 10.1002/dvdy.24285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauchunas A.R., Sackton K.L., Wolfner M.F. Phospho-regulation pathways during egg activation in Drosophila melanogaster. Genetics. 2013;195:171–180. doi: 10.1534/genetics.113.150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist U., Björndahl L., Kjellberg S. Sperm nuclear zinc, chromatin stability, and male fertility. Scanning Microsc. 1987;1:1241–1247. [PubMed] [Google Scholar]
- Leader D.P., Krause S.A., Pandit A., Davies S.A., Dow J.A.T. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res. 2017;46:D809–D815. doi: 10.1093/nar/gkx976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J.M., Bratu D.P. Drosophila melanogaster oogenesis: an overview. In: Bratu D.P., McNeil G.P., editors. Drosophila Oogenesis: Methods and Protocols. Springer New York; 2015. pp. 1–20. [Google Scholar]
- Mendoza A.D., Woodruff T.K., Wignall S.M., O’Halloran T.V. Zinc availability during germline development impacts embryo viability in Caenorhabditis elegans. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017;191:194–202. doi: 10.1016/j.cbpc.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- Page A.W., Orr-Weaver T.L. Activation of the meiotic divisions in Drosophila oocytes. Dev. Biol. 1997;183:195–207. doi: 10.1006/dbio.1997.8506. [DOI] [PubMed] [Google Scholar]
- Pepling M.E., Spradling A.C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Percival S.S., Layden-Patrice M. HL-60 cells can be made copper deficient by incubating with tetraethylenepentamine. J. Nutr. 1992;122:2424–2429. doi: 10.1093/jn/122.12.2424. [DOI] [PubMed] [Google Scholar]
- Que E.L., Bleher R., Duncan F.E., Kong B.Y., Gleber S.C., Vogt S., Chen S., Garwin S.A., Bayer A.R., Dravid V.P. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat. Chem. 2015;7:130–139. doi: 10.1038/nchem.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que E.L., Duncan F.E., Bayer A.R., Philips S.J., Roth E.W., Bleher R., Gleber S.C., Vogt S., Woodruff T.K., O’Halloran T.V. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr. Biol. 2017;9:135–144. doi: 10.1039/c6ib00212a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que E.L., Duncan F.E., Lee H.C., Hornick J.E., Vogt S., Fissore R.A., O’Halloran T.V., Woodruff T.K. Bovine eggs release zinc in response to parthenogenetic and sperm-induced egg activation. Theriogenology. 2019;127:41–48. doi: 10.1016/j.theriogenology.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohani N., Hurrell R., Kelishadi R., Schulin R. Zinc and its importance for human health: an integrative review. J. Res. Med. Sci. 2013;18:144–157. [PMC free article] [PubMed] [Google Scholar]
- Sartain C.V., Wolfner M.F. Calcium and egg activation in Drosophila. Cell Calcium. 2013;53:10–15. doi: 10.1016/j.ceca.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigel-Röntgen M. Chapter nine - the families of zinc (SLC30 and SLC39) and copper (SLC31) transporters. In: Bevensee M., editor. Current Topics in Membranes, Exchangers. Academic Press; 2014. pp. 321–355. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Yoshida N., Suzuki E., Okuda E., Perry A.C.F. Full-term mouse development by abolishing Zn2+-dependent metaphase II arrest without Ca2+ release. Development. 2010;137:2659–2669. doi: 10.1242/dev.049791. [DOI] [PubMed] [Google Scholar]
- Swann K., Lai F.A. Egg activation at fertilization by a soluble sperm protein. Physiol. Rev. 2016;96:127–149. doi: 10.1152/physrev.00012.2015. [DOI] [PubMed] [Google Scholar]
- Takeo S., Hawley R.S., Aigaki T. Calcineurin and its regulation by Sra/RCAN is required for completion of meiosis in Drosophila. Dev. Biol. 2010;344:957–967. doi: 10.1016/j.ydbio.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Takeo S., Tsuda M., Akahori S., Matsuo T., Aigaki T. The calcineurin regulator sra plays an essential role in female meiosis in Drosophila. Med. Eng. Phys. 2006;16:1435–1440. doi: 10.1016/j.cub.2006.05.058. [DOI] [PubMed] [Google Scholar]
- Tejeda-Guzmán C., Rosas-Arellano A., Kroll T., Webb S.M., Barajas-Aceves M., Osorio B., Missirlis F. Biogenesis of zinc storage granules in Drosophila melanogaster. J. Exp. Biol. 2018;221:jeb168419. doi: 10.1242/jeb.168419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Anthony K., Neuberger T., Diaz F.J. Preconception zinc deficiency disrupts postimplantation fetal and placental development in Mice1. Biol. Reprod. 2014;90:83. doi: 10.1095/biolreprod.113.113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Diaz F.J. Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development. Dev. Biol. 2013;376:51–61. doi: 10.1016/j.ydbio.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Diaz F.J. Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology. 2012;153:873–886. doi: 10.1210/en.2011-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuhiro K., Dean J. Glycan-independent gamete recognition triggers egg zinc sparks and ZP2 cleavage to prevent polyspermy. Dev. Cell. 2018;46:627–640.e5. doi: 10.1016/j.devcel.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S.L., Welte M.A. In-vivo centrifugation of Drosophila embryos. J. Vis. Exp. 2010;40:2005. doi: 10.3791/2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stetina J.R., Orr-Weaver T.L. Developmental control of oocyte maturation and egg activation in metazoan models. Cold Spring Harb. Perspect. Biol. 2011;3:1–19. doi: 10.1101/cshperspect.a005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepiskoposyan H., Egli D., Fergestad T., Selvaraj A., Treiber C., Multhaup G., Georgiev O., Schaffner W. Transcriptome response to heavy metal stress in Drosophila reveals a new zinc transporter that confers resistance to zinc. Nucleic Acids Res. 2006;34:4866–4877. doi: 10.1093/nar/gkl606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York-Andersen A.H., Parton R.M., Bi C.J., Bromley C.L., Davis I., Weil T.T. A single and rapid calcium wave at egg activation in Drosophila. Biol. Open. 2015;4:553–560. doi: 10.1242/bio.201411296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Ahmed-Braimah Y., Goldberg M.L., Wolfner M.F. Calcineurin-dependent protein phosphorylation changes during egg activation in Drosophila melanogaster. Mol. Cell Proteomics. 2018;18:S145–S158. doi: 10.1074/mcp.RA118.001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Duncan F.E., Que E.L., O’Halloran T.V., Woodruff T.K., O’Halloran T.V., Woodruff T.K. The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development. Sci. Rep. 2016;6:22772. doi: 10.1038/srep22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The XFM raw data are available at https://drive.google.com/drive/folders/1O9wDQbawoZ2Ytp9LwcLB2hCFDFwATuXe?usp=sharing; R codes used for analysis and plotting are available at https://github.com/WolfnerLab/zinc_reproduction.