Abstract
Left bundle branch block (LBBB) is associated with improved outcome after cardiac resynchronisation therapy (CRT). One historical presumption of LBBB has been that the underlying pathophysiology involved diffuse disease throughout the distal conduction system. The ability to normalize wide QRS patterns with His bundle pacing (HBP) has called this notion into question. The determination of LBBB pattern is conventionally made by assessment of surface 12-lead ECGs and can include patients with and without conduction block, as assessed by invasive electrophysiology study (EPS). During a novel extension of the classical EPS to involve left-sided recordings, we found that conduction block associated with the LBBB pattern is most often proximal, usually within the left-sided His fibres, and these patients are the most likely to demonstrate QRS correction with HBP for resynchronisation. Patients with intact Purkinje activation and intraventricular conduction delay are less likely to benefit from HBP. Future EPS are required to determine the impact of newer approaches to conduction system pacing, including intraseptal or left ventricular septal pacing. Left-sided EPS has the potential to refine patient selection in CRT trials and may be used to physiologically phenotype distinct conduction patterns beyond LBBB pattern.
Keywords: Cardiac resynchronisation therapy, left bundle branch block, His bundle, septal activation, conduction block, biventricular pacing, electrophysiology
In 1970, Durrer et al. recorded the total excitation of the human heart with 870 electrodes and confirmed the first 10 ms of left ventricular (LV) activation as trifascicular in nature.[1] The rapid and synchronous activation of the LV through the specialised His–Purkinje network is highly intricate and efficient, preserving normal physiological coupling between electrical excitation and mechanical contraction. When normal transit through the His–Purkinje system is disrupted, stereotypic conduction block patterns are manifested on the 12-lead surface ECG (e.g. fascicular blocks or bundle branch blocks). These abnormal electrical activation patterns, particularly the left bundle branch block (LBBB) pattern, are associated with the presence, development or worsening of cardiomyopathy, and leads to increased risk of subsequent morbidity and mortality.
Cardiac resynchronisation therapy (CRT) via biventricular pacing has been established as the mainstay electrical pacing modality to reverse the deleterious effects of electromechanical dyssynchrony, with significant reductions in mortality and heart failure (HF) hospitalisation for patients with wide QRS, as shown in multiple randomised controlled trials.[2–7] However, despite these significant benefits, up to one-third of patients do not improve after biventricular pacing, and the overall rate of response from traditional biventricular pacing has remained stagnant despite technical improvements in lead delivery and site selection.[8–10] During the past decade, we have gained a deeper mechanistic understanding of the benefits of biventricular pacing. Research has shown that patients with LBBB are the most likely to benefit from CRT, and that patients without significant LV conduction delay, particularly those with right bundle branch (RBBB) or non-specific intraventricular conduction delay (IVCD), derive little to no benefit from biventricular pacing.[11–14] Non-response is not necessarily a failure of biventricular pacing, but rather a failure in appropriate patient selection. The physiology of conduction system disease is highly individualised and requires therapy directed at the patient’s underlying pathophysiology; traditional LV lead placement into an available tributary of the coronary sinus might not meet the needs of an individual patient. Indeed, the goal of CRT more broadly should be complete physiological resynchronisation utilising the His–Purkinje system, if possible. Identifying patients who might achieve physiological resynchronisation, however, can be challenging. In this review, we discuss the need to return to the ‘renaissance’ of electrophysiology (EP) when His bundle and electrical conduction system recordings were the focus of invasive study.[15] In CRT, a precise diagnosis of the level and extent of conduction system pathology could improve technology selection, lead delivery and outcomes of physiological pacing.
Recruitment of the Conduction System: His Bundle Pacing
Biventricular pacing achieves resynchronisation via non-physiological fusion of a right ventricular apical-paced wavefront and an epicardial wavefront from the basal lateral LV (and some newer techniques attempt left-sided fusion with native right-sided His-Purkinje activation). His bundle pacing (HBP) is a promising method to achieve electromechanical resynchronisation, as pacing utilising this strategy recruits the intrinsic specialised conduction system.[16,17] Relative to traditional dual-chamber or biventricular pacing, HBP has been shown to preserve intrinsic activation in patients with narrow QRS,[18] as well as restore a narrow QRS in patients with LBBB and RBBB patterns.[19–21]
Until recently, the underlying mechanism of how HBP (delivered proximally in the conduction system) corrects LBBB patterns (a distal defect) had not been well understood. Based on fine anatomic dissection studies of human and animal hearts in the 1960s and early 1970s, James and Sherf proposed a concept of sinoventricular conduction, in which impulses originating in the sinus node were predestined to specific locations in the ventricle.[22] They also identified longitudinal separation of Purkinje strands with collagen within the His bundle, and introduced the concept that proximal lesions might impact more distal conduction.[23]
In the late 1970s, Narula demonstrated normalisation of LBBB patterns with stimulation of the distal His bundle, and based on James and Sherf’s work, interpreted these findings to prove the existence of longitudinally dissociated fibres with asynchronous conduction due to a discrete lesion or altered refractoriness within the His bundle.[24] At the same time, El-Sherif et al. provided further experimental and clinical observations of the normalisation of wide QRS (both RBBB and LBBB) with distal HBP, which was also interpreted as evidence of functional longitudinal dissociation of conduction from a pathological His bundle resulting in distal asynchronous conduction.[25] However, these seminal observations and studies did not investigate the left-sided conduction system distal to the His bundle. It is important to emphasise that, according to this 40-year-old theory of functional longitudinal dissociation of the His bundle, conduction through the left bundle branch was theoretically proposed as remaining intact without actual conduction block, as the lesion within the His itself is sufficient to create sufficient conduction delay to create a bundle branch block pattern.
Variability in LBBB Pattern Definitions
Historically, wide (≥120 ms) QRS patterns with dominant S-waves in lead V1 have been aggregated into the broad categorisation of LBBB pattern. However, not all conduction block patterns seen on the surface ECG are indicative of the same pathology. As such, LBBB patterns include both subtypes of those without discrete conduction block and those with ‘true’ or complete conduction block into or within the left bundle. In this regard, ‘block’ is a semantic error, as many patients might have intact activation of the Purkinje system.
A prevailing definition of the LBBB pattern was developed by the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology/American College of Cardiology Foundation/Heart Rhythm Society (AHA/ACCF/HRS) in 2009.
The LBBB pattern required a QRS ≥120 ms with a broad notched or slurred R-wave in leads I, aVL, V5 and V6.[26] The cut-off of 120 ms was primarily historical and was revisited by Strauss and colleagues in 2011, who proposed a cut-off of ≥140 ms in men and ≥130 ms in women, along with the requirements of a QS or RS in leads V1–V2, and mid-QRS notching or slurring in two or more of leads V1, V2, V5, V6, I and aVL.[27] The outcomes of CRT via biventricular pacing have been shown to vary based on how left LBBB is defined,[28–30] which illustrates the need to optimise the phenotyping of patients with wide QRS eligible for CRT. Multiple ECG criteria have been assessed, but without a ‘gold standard’ of determination of whether or not block was present. The interpretation of notching and slurring within the QRS is also highly subjective.
Direct Recordings of Activation Patterns During LBBB: A New Gold Standard?
To further investigate the theory of longitudinal dissociation in the His bundle, we commenced EP testing to delineate the activation patterns of the proximal left conduction system with multielectrode catheters in patients presenting for cardiac resynchronisation or substrate mapping for ventricular tachycardia ablation.[31] Prior mapping studies performed to characterise LBBB patterns focused on myocardial breakout locations.[32–34] We hypothesised that lesions within the His bundle could be more evident with bilateral His recordings,where the left-sided His might have discrepant or delayed timing relative to the right-sided His bundle activation (presenting as a ‘split-His’ with observable interhisian delay).
To our surprise, we did not observe any differences in the His bundle activation recorded from the right and left side, and split His electrograms were rarely observed in patients with LBBB pattern. Rather, these findings were only present in patients who served as narrow QRS complex controls. Importantly, we observed complete disruption and absence of conduction in the left bundle with detailed contact mapping with multielectrode catheters. Of note, the conduction block site was frequently proximal to the left bundle, as the last recorded pre-potential was consistent with block within the left-sided His, and timing coincided with right-sided His activation with a local atrial component (Figure 1). To the best of our knowledge, this was the first observation of the focal ‘left intrahisian’ conduction block, where the central pathology exposed was a conduction defect into the left bundle, rather than within the left bundle.
Figure 1: Left Intrahisian Left Bundle Branch Block.
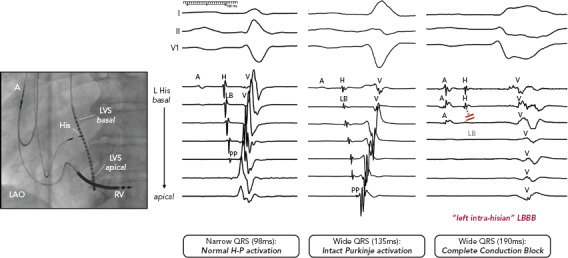
Intracardiac subclassification of LBBB patterns with intact Purkinje activation evidenced by continuous presystolic activation of specialised conduction system potentials with apical to basal ventricular activation in contrast to complete conduction block proximal to the left bundle at the level of the left-sided His recording. An atrial component is recorded at the site of the conduction block, indicating high and proximal pathology location. The ventricular activation is non-physiological, with basal to apical passive activation. LAO = left anterior oblique; LBBB = left bundle branch block; LVS = left ventricular septum; RV = right ventricle. Source: Upadhyay et al. 2019.[31] Reproduced with permission from Wolters Kluwer Health. The Creative Commons license does not apply to this content. Use of the material in any format is prohibited without written permission from the publisher, Wolters Kluwer Health. Please contact permissions@lww.com for further information.
In contrast, in patients with either narrow QRS or RBBB pattern, intact Purkinje activation (IPA) was noted on the left side of the septum. In our study, 11 patients with narrow QRS and five patients with RBBB served as controls for patients with LBBB pattern at baseline. The absolute difference between the earliest and latest recorded ventricular maximum peak electrogram on our left septal recording catheter (ΔLV time) and transseptal conduction time for patients with narrow QRS or RBBB was similar to that of patients with IVCD, and in marked contrast to patients with complete conduction block and ‘true’ LBBB.
The findings of complete conduction block at the level of the left-sided His fibres or the proximal LBBB are consistent with the theory of longitudinal dissociation, in that the inferred site of pathology is a proximal lesion within the branching His bundle, but differs significantly from this proposed theory because left bundle activation was not asynchronous relative to the right bundle, but completely blocked (Figure 2).
Figure 2: Comparing Theory of Longitudinal Dissociation with Finding of Conduction Block.
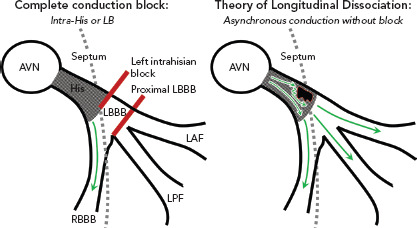
Comparison of complete conduction block observed with dedicated electrophysiology study of the left septum compared to the theory of longitudinal dissociation with intact distal conduction (green arrows), but asynchronous conduction. In both models, the site of the conduction system disease is focal and localised to the His fibres, but complete conduction block is not a component of the theory of functional delay from longitudinal dissociation of the His bundle. AD = anterior division of left bundle; AVN = atrioventricular node; LB = left bundle; LBBB = left bundle branch block; RBBB = right bundle branch block; LAF = left anterior fascicle; LPF = left posterior fascicle.
Importantly, we observed presystolic recruitment of latent Purkinje potentials in patients with complete conduction block in the left bundle during His corrective pacing (Figure 3). These findings provide direct evidence of the mechanism of QRS narrowing with HBP, where complete conduction block is circumvented by a pacing stimulus that sufficiently captures the conduction system distal to the site of discrete block. Similar to the controls or RBBB patients noted earlier, patients without complete conduction block were observed to have IPA, and these patients uniformly failed attempts at His corrective pacing (Figure 4).
Figure 3: Complete Conduction Block with Recruitment of Latent Purkinje Potentials During His Bundle Pacing with QRS Correction.
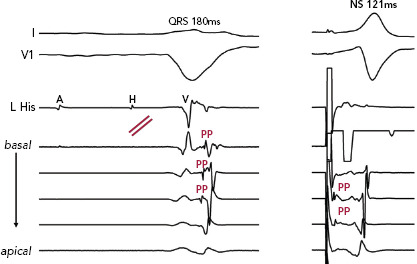
Complete conduction block at the level of the left-sided His without any left bundle recordings suggestive of block proximal to the left bundle. The PP are retrograde and passive after ventricular activation. With His bundle stimulation, QRS narrowing from 180 to 121 ms is observed. Non-selective His bundle capture is demonstrated by presystolic recruitment of the latent PP immediately after the pacing impulse. Normal physiological activation of the local ventricular electrograms is restored from apical to basal. NS = non-selective; PP = Purkinje potentials.
Figure 4: Failure to Correct QRS in Patient with Intact Purkinje Activation During Right or Left-sided His Bundle Pacing.
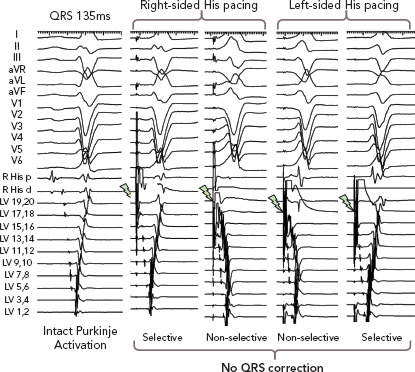
Inability to correct wide QRS in a patient with LBBB with intact Purkinje activation with pacing from both right- and left-side His bundle recording sites. Selective and non-selective stimulation of the His bundle fails to narrow the QRS width of 135 ms. LV = left ventricular. Source: Upadhyay et al. 2019.[31] Reproduced with permission from Wolters Kluwer Health. The Creative Commons license does not apply to this content. Use of the material in any format is prohibited without written permission from the publisher, Wolters Kluwer Health. Please contact permissions@lww.com for further information.
Lessons from the His-SYNC Trial
The His-SYNC pilot trial was the first multicentre randomised trial to evaluate corrective HBP in direct comparison with biventricular pacing.[35] At the time of study conception, it was not known if IVCD would fare better or worse with HBP, and therefore, a broad definition of LBBB pattern (according to the 2009 AHA/ACCF/HRS consensus criteria) was used for inclusion. With a large crossover rate (48% from His CRT to biventricular pacing), the intention-to-treat outcomes were comparable between the two groups with regard to echocardiographic response, and His corrective pacing failed to reach statistical superiority, defined as a difference of 10% in ejection fraction.
In a post-hoc analysis, 50% of patients (n=5) who crossed over to biventricular pacing from His pacing had IVCD patterns that could not be corrected by HBP.[36] Perhaps the most important lesson learned from the His-SYNC pilot trial was that patients with IVCD should be excluded from enrolment in future HBP and conduction system pacing studies for CRT. Indeed, these patients appear to be best suited to traditional biventricular devices, although outcomes for patients with IVCD are known to be less favourable than those of patients with LBBB. Confirmation of intact Purkinje activation with left-sided conduction system mapping would potentially assist in patient selection to exclude patients with IVCD and apparent LBBB pattern on surface ECG. In addition, contact mapping can also afford assessment of His corrective thresholds prior to lead deployment. Indeed, even for patients with complete conduction block beyond the left-sided His fibres and in the proximal left bundle branch, higher outputs are generally required to achieve QRS correction. We found that patients with complete conduction block within the left bundle had lower rates of QRS correction compared to those with left intrahisian block (62% versus 94%, respectively; Figure 5).
Figure 5: Sites of Conduction Block in Patients with Left Bundle Branch Block Pattern with Rate of Response to Corrective His Bundle Pacing.
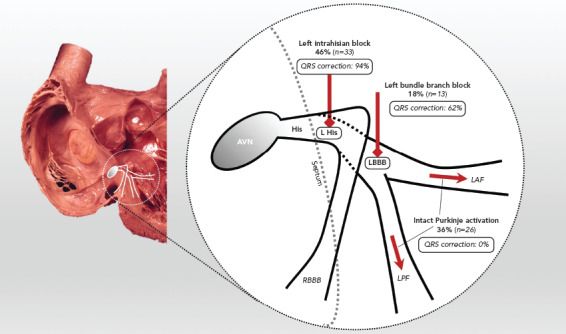
In this schematic of the proximal conduction system, the distribution of septal conduction among patients with LBBB patterns is shown, along with the location of block, if present. The septum is shown (dashed) to represent the location of the right-sided His fibres as they penetrate the central fibrous body, which then give rise to the left-sided His fibers (distal portion and branching bundle). In patients with complete conduction block (CCB), block was localised to the left-sided His fibres most commonly, and this location was most amenable to corrective His bundle pacing. Less commonly, CCB was found distal to the His recording site, at locations in which an atrial electrogram was not recorded. These locations were less amenable to corrective His bundle pacing and were anatomically consistent with block in the distal branching bundle or proximal left bundle-branch. The remainder of the patients with LBBB pattern did not demonstrate CCB. Assessment of local ventricular electrograms showed intact Purkinje activation, and the QRS was wide, most likely because of conduction slowing more distally. AVN = atrioventricular node; LBBB = left bundle branch block; LPF = left posterior fascicle; RBBB = right bundle branch block. Photograph courtesy of the University of California, Los Angeles, Cardiac Arrhythmia Center, Wallace A McAlpine, MD, collection; reproduced with permission from K Shivkumar, MD, PhD. Source: Upadhyay et al. 2019.[31] Reproduced with permission from Wolters Kluwer Health. The Creative Commons license does not apply to this content. Use of the material in any format is prohibited without written permission from the publisher, Wolters Kluwer Health. Please contact permissions@lww.com for further information.
Routine Left-sided Electrophysiological Testing for CRT
The most rigorous and physiological method to evaluate corrective HBP prospectively would be to invasively confirm disease within the left-sided His fibres or proximal LBBB with EP prior to randomisation. The attendant risk of a retrograde transaortic approach needs to be carefully assessed prior to implementing routine left-sided EP. Although this risk is unlikely to be higher than a routine diagnostic left heart catheterisation with coronary angiography (stroke <1%), there could be greater risk in patients with extensive aortic arch atheromatous calcification or disease.[37]
The diagnostic duodecapolar catheter that we use (Livewire, 2-2-2; Abbott) is routinely employed for substrate mapping during ventricular tachycardia ablation, and should theoretically carry less risk for thromboembolism than catheters used for diagnostic angiography, as there is no lumen. Systemic heparinisation should be considered in patients with extended LV dwell time, and could also be associated with increased risk of pocket haematoma during implantation. Emerging data suggest that patients could safely undergo device implantation during uninterrupted anticoagulation, including warfarin or non-vitamin K antagonists (e.g. apixaban, rivaroxaban or dabigatran), and singledose heparin to facilitate EP could be beneficial.[38,39]
With regard to non-invasive evaluation, there is a need to develop novel criteria to determine the presence of complete conduction block into the left bundle, as these patients are more likely to benefit from resynchronisation strategies (either biventricular pacing or HBP). The Strauss criteria has widely been recognised to be associated with better outcome prognostication after biventricular pacing. However, we found that 39% of patients with intact Purkinje activation demonstrated by left septal mapping met the Strauss criteria.[40] The most helpful surface ECG criterion identified was the presence of mid-QRS notching, which had an excellent negative prediction value of 100%. It is worth noting that the observation of notching could be associated with a degree of subjectivity. Indeed, the literature often uses the terms ‘slurring’ and ‘notching’ interchangeably, when these likely reflect distinct underlying EP phenomena. Also not explicitly mentioned in the guidelines are patients demonstrating a ‘plateau’ within the QRS complex or whether this is a comparable observation to notching or slurring. Future work should be directed towards analysing the specific features of the QRS complex, which are most likely to be associated with underlying complete conduction block, and contemporary articulation of the guidelines should incorporate direction on how to classify based on these features more explicitly.
There are recent reports that have successfully utilised artificial intelligence to detect subtle patterns in digitally acquired ECG data.[41] While these techniques appear quite promising, approaches, such as convolutional neural networks, require large datasets (ideally in the hundreds of thousands) for algorithm training and robustness, and availability of this gold standard set of patients remains limited.
Indeed, surface ECG recordings might always have limitations that will yield a diagnostic accuracy that falls short of direct recordings, as broadly applied criteria might not account for variability in chamber size, wall thickness, anatomic rotation and scar location. More recently, the development of high-density surface ECG assessment utilising either a vest or a belt has been proposed as a means to overcome some of the conventional limitations of the 12 lead.[42,43] Septal activation, however, cannot be directly assessed with either technique, as both approaches predominantly utilise epicardial voltage assessment to construct activation maps. Theoretically, patients with significant leftsided IVCD could be miscategorised as having LBBB. These patients might still benefit from biventricular pacing, but would be unlikely to respond to HBP for resynchronisation. With the proposed His-SYNC II trial, patients with IVCD will be specifically excluded, and the role of left-sided His recordings and invasive diagnostic EP testing remains to be determined in the planned protocol. Echocardiography-based measures of electromechanical delay, including apical rocking, systolic stretch index or septal flash, might also be beneficial. The integration of echocardiography with ECG measures in future studies is of ongoing interest and debate.
Predictions and Future Studies
The evolution and re-emergence of HBP provides more options for CRT and marks a return to much needed emphasis on the physiology and pathophysiology of the conduction system. Much remains to be learned about the complex nature of the highly specialised His–Purkinje network. In patients with RBBB who traditionally do not benefit from biventricular pacing, we believe that HBP might emerge as a first-line therapy. Preliminary data are promising and prospective studies are necessary to confirm these findings.[20] In patients with IVCD, it is our opinion that HBP and conduction system pacing for resynchronisation should not be considered as a monotherapeutic option, and diagnostic EP could be helpful to quickly exclude a patient’s candidacy, thereby minimising crossovers in future trials.
Importantly, HBP and conduction system pacing for resynchronisation are currently used in the case of failed biventricular pacing or in the setting of a clinical study, as traditional biventricular pacing has been clearly shown to have improved survival, which has not yet been demonstrated in randomised controlled trials of HBP.[19] Intraseptal or LV septal fixation techniques could have the potential to correct distal conduction block in the left bundle or in cases where HBP for resynchronisation is associated with high pacing output.[44–46] Presently, more data are available on patients with narrow QRS utilising these approaches, and consensus in definitions of intraseptal pacing with and without left conduction system capture is needed.[47–51] In addition, invasive EP diagnostic recordings to delineate left septal activation during intraseptal pacing are likely to provide deeper physiological insights into these and other emergent strategies for conduction system pacing.
Clinical Perspective
Left bundle branch (LBBB) is well recognised to be associated with favorable outcome after CRT.
The mechanism of benefit for LBBB with CRT is through addressing lateral left ventricular delay by biventricular pacing, or through corrective His bundle pacing which engages latent His–Purkinje fibres and restores physiologic activation.
LBBB is usually identified through use of surface 12-lead ECG, which lacks specificity in identifying patients with complete conduction block.
Invasive electrophysiology (EP) study can quickly ascertain the presence of conduction block through evaluation of left ventricular septal activation patterns.
There may be utility in pursuing left-sided EP study to better differentiate conduction patterns in order to tailor therapy selection.
References
- 1.Durrer D, van Dam RT, Freud GE et al. Total excitation of the isolated human heart. Circulation. 1970;41:899–912. doi: 10.1161/01.cir.41.6.899. [DOI] [PubMed] [Google Scholar]
- 2.Cazeau S, Leclercq C, Lavergne T et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–80. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 3.Auricchio A, Stellbrink C, Sack S et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39:2026–33. doi: 10.1016/s0735-1097(02)01895-8. [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT, Fisher WG, Smith AL et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 5.Young JB, Abraham WT, Smith AL et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 6.Bristow MR, Saxon LA, Boehmer J et al. Cardiacresynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Daubert JC, Erdmann E et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 8.Daubert C, Behar N, Martins RP et al. Avoiding non-responders to cardiac resynchronization therapy: a practical guide. Eur Heart J. 2017;38:1463–72. doi: 10.1093/eurheartj/ehw270. [DOI] [PubMed] [Google Scholar]
- 9.European Heart Rhythm Association, European Society of Cardiology, Heart Rhythm Society, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace. 2012;14:1236–86. doi: 10.1093/europace/eus222. [DOI] [PubMed] [Google Scholar]
- 10.Fornwalt BK, Sprague WW, BeDell P et al. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation. 2010;121:1985–91. doi: 10.1161/CIRCULATIONAHA.109.910778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunnington C, Kwok CS, Satchithananda DK et al. Cardiac resynchronisation therapy is not associated with a reduction in mortality or heart failure hospitalisation in patients with non-left bundle branch block QRS morphology: meta-analysis of randomised controlled trials. Heart. 2015;101:1456–62. doi: 10.1136/heartjnl-2014-306811. [DOI] [PubMed] [Google Scholar]
- 12.Bilchick KC, Kamath S, DiMarco JP, Stukenborg GJ. Bundlebranch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation. 2010;122:2022–30. doi: 10.1161/CIRCULATIONAHA.110.956011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zareba W, Klein H, Cygankiewicz I et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123:1061–72. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 14.Peterson PN, Greiner MA, Qualls LG et al. QRS duration, bundle-branch block morphology, and outcomes among older patients with heart failure receiving cardiac resynchronization therapy. JAMA. 2013;310:617–26. doi: 10.1001/jama.2013.8641. [DOI] [PubMed] [Google Scholar]
- 15.Scherlag BJ, Kosowsky BD, Damato AN. A technique for ventricular pacing from the His bundle of the intact heart. J Appl Physiol. 1967;22:584–7. doi: 10.1152/jappl.1967.22.3.584. [DOI] [PubMed] [Google Scholar]
- 16.Vijayaraman P, Dandamudi G, Zanon F et al. Permanent His bundle pacing: recommendations from a Multicenter His Bundle Pacing Collaborative Working Group for standardization of definitions, implant measurements, and follow-up. Heart Rhythm. 2018;15:460–8. doi: 10.1016/j.hrthm.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Vijayaraman P, Chung MK, Dandamudi G et al. His bundle pacing. J Am Coll Cardiol. 2018;72:927–47. doi: 10.1016/j.jacc.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Abdelrahman M, Subzposh FA, Beer D et al. Clinical outcomes of His bundle pacing compared to right ventricular pacing. J Am Coll Cardiol. 2018;71:2319–30. doi: 10.1016/j.jacc.2018.02.048. [DOI] [PubMed] [Google Scholar]
- 19.Sharma PS, Dandamudi G, Herweg B et al. Permanent Hisbundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm. 2018;15:413–20. doi: 10.1016/j.hrthm.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Sharma PS, Naperkowski A, Bauch TD et al. Permanent His bundle pacing for cardiac resynchronization therapy in patients with heart failure and right bundle branch block. Circ Arrhythm Electrophysiol. 2018;11:e006613. doi: 10.1161/CIRCEP.118.006613. [DOI] [PubMed] [Google Scholar]
- 21.Lustgarten DL, Crespo EM, Arkhipova-Jenkins I et al. Hisbundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: a crossover design comparison. Heart Rhythm. 2015;12:1548–57. doi: 10.1016/j.hrthm.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 22.Sherf L, James TN. A new electrocardiographic concept: synchronized sinoventricular conduction. Dis Chest. 1969;55:127–40. doi: 10.1378/chest.55.2.127. [DOI] [PubMed] [Google Scholar]
- 23.James TN, Sherf L. Fine structure of the His bundle. Circulation. 1971;44:9–28. doi: 10.1161/01.cir.44.1.9. [DOI] [PubMed] [Google Scholar]
- 24.Narula OS. Longitudinal dissociation in the His bundle. Bundle branch block due to asynchronous conduction within the His bundle in man. Circulation. 1977;56:996–1006. doi: 10.1161/01.cir.56.6.996. [DOI] [PubMed] [Google Scholar]
- 25.El-Sherif N, Amay YLF, Schonfield C et al. Normalization of bundle branch block patterns by distal His bundle pacing. Clinical and experimental evidence of longitudinal dissociation in the pathologic His bundle. Circulation. 1978;57:473–83. doi: 10.1161/01.cir.57.3.473. [DOI] [PubMed] [Google Scholar]
- 26.Surawicz B, Childers R, Deal BJ et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e235–40. doi: 10.1161/CIRCULATIONAHA.108.191095. [DOI] [PubMed] [Google Scholar]
- 27.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–34. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Caputo ML, van Stipdonk A, Illner A et al. The definition of left bundle branch block influences the response to cardiac resynchronization therapy. Int J Cardiol. 2018;269:165–9. doi: 10.1016/j.ijcard.2018.07.060. [DOI] [PubMed] [Google Scholar]
- 29.Perrin MJ, Green MS, Redpath CJ et al. Greater response to cardiac resynchronization therapy in patients with true complete left bundle branch block: a PREDICT substudy. Europace. 2012;14:690–5. doi: 10.1093/europace/eur381. [DOI] [PubMed] [Google Scholar]
- 30.Jastrzebski M, Kukla P, Kisiel R et al. Comparison of four LBBB definitions for predicting mortality in patients receiving cardiac resynchronization therapy. Ann Noninvasive Electrocardiol. 2018;23:e12563. doi: 10.1111/anec.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Upadhyay GA, Cherian T, Shatz DY et al. Intracardiac delineation of septal conduction in left bundle-branch block patterns. Circulation. 2019;139:1876–88. doi: 10.1161/CIRCULATIONAHA.118.038648. [DOI] [PubMed] [Google Scholar]
- 32.Auricchio A, Fantoni C, Regoli F et al. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109:1133–9. doi: 10.1161/01.CIR.0000118502.91105.F6. [DOI] [PubMed] [Google Scholar]
- 33.Vassallo JA, Cassidy DM, Marchlinski FE et al. Endocardial activation of left bundle branch block. Circulation. 1984;69:914–23. doi: 10.1161/01.cir.69.5.914. [DOI] [PubMed] [Google Scholar]
- 34.Derval N, Duchateau J, Mahida S et al. Distinctive left ventricular activations associated with ECG pattern in heart failure patients. Circ Arrhythm Electrophysiol. 2017;10:e005073. doi: 10.1161/CIRCEP.117.005073. [DOI] [PubMed] [Google Scholar]
- 35.Upadhyay GA, Vijayaraman P, Nayak HM et al. His corrective pacing or biventricular pacing for cardiac resynchronization in heart failure. J Am Coll Cardiol. 2019;74:157–9. doi: 10.1016/j.jacc.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 36.Upadhyay GA, Vijayaraman P, Nayak HM et al. On-treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: a secondary analysis of His-SYNC. Heart Rhythm. 2019;16:1797–807. doi: 10.1016/j.hrthm.2019.05.009l. [DOI] [PubMed] [Google Scholar]
- 37.de Bono D. Complications of diagnostic cardiac catheterisation: results from 34,041 patients in the United Kingdom confidential enquiry into cardiac catheter complications. The Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London. Br Heart J. 1993;70:297–300. doi: 10.1136/hrt.70.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birnie DH, Healey JS, Wells GA et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368:2084–93. doi: 10.1056/NEJMoa1302946. [DOI] [PubMed] [Google Scholar]
- 39.Birnie DH, Healey JS, Wells GA et al. Continued vs. interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thromboembolic events (BRUISE CONTROL-2) Eur Heart J. 2018;39:3973–9. doi: 10.1093/eurheartj/ehy413. [DOI] [PubMed] [Google Scholar]
- 40.Tung S, Lemaitre J. His bundle pacing: in pursuit of the “sweet spot”. Pacing Clin Electrophysiol. 2015;38:537–9. doi: 10.1111/pace.12604. [DOI] [PubMed] [Google Scholar]
- 41.Attia ZI, Kapa S, Lopez-Jimenez F et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019;25:70–4. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 42.Ploux S, Lumens J, Whinnett Z et al. Noninvasive electrocardiographic mapping to improve patient selection for cardiac resynchronization therapy: beyond QRS duration and left bundle branch block morphology. J Am Coll Cardiol. 2013;61:2435–43. doi: 10.1016/j.jacc.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 43.Johnson WB, Vatterott PJ, Peterson MA et al. Body surface mapping using an ECG belt to characterize electrical heterogeneity for different left ventricular pacing sites during cardiac resynchronization: relationship with acute hemodynamic improvement. Heart Rhythm. 2017;14:385–91. doi: 10.1016/j.hrthm.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 44.Huang W, Su L, Wu S et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol. 2017;33:1736e1–3. doi: 10.1016/j.cjca.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Rademakers LM, van Hunnik A, Kuiper M et al. A possible role for pacing the left ventricular septum in cardiac resynchronization therapy. JACC Clin Electrophysiol. 2016;2:413–22. doi: 10.1016/j.jacep.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Huang W, Chen X, Su L et al. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16:1791–6. doi: 10.1016/j.hrthm.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Mafi-Rad M, Luermans JG, Blaauw Y et al. Feasibility and acute hemodynamic effect of left ventricular septal pacing by transvenous approach through the interventricular septum. Circ Arrhythm Electrophysiol. 2016;9:e003344. doi: 10.1161/CIRCEP.115.003344. [DOI] [PubMed] [Google Scholar]
- 48.Vijayaraman P, Subzposh FA, Naperkowski A et al. Prospective evaluation of feasibility, electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm. 2019;16:1774–82. doi: 10.1016/j.hrthm.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Huang J, Qi Y et al. Cardiac resynchronization therapy by left bundle branch area pacing in patients with heart failure and left bundle branch block. Heart Rhythm. 2019;16:1783–90. doi: 10.1016/j.hrthm.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Li H, Ma W et al. Permanent left bundle branch area pacing for atrioventricular block: feasibility, safety, and acute effect. Heart Rhythm. 2019;16:1766–73. doi: 10.1016/j.hrthm.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Chen K, Dai Y et al. Left bundle branch pacing for symptomatic bradycardia: implant success rate, safety, and pacing characteristics. Heart Rhythm. 2019;16:1758–65. doi: 10.1016/j.hrthm.2019.05.014. [DOI] [PubMed] [Google Scholar]


