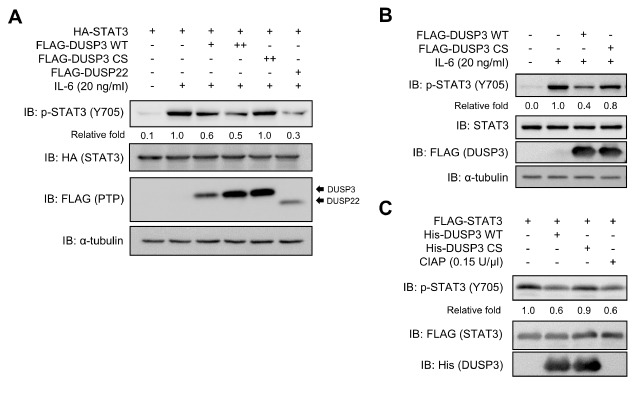
Fig. 3.
Direct dephosphorylation of p-STAT3 by DUSP3 both in cell and in vitro. (A) HEK 293 cells were co-transfected with FLAG-PTPs and HA-STAT3. DUSP22 was used as positive control. (B) SK-Hep1 cells were transfected with FLAG-DUSP3 WT or CS. (A, B) After transfection, cells were starved with serum-free medium for 12 (HEK 293) or 24 h (SK-Hep1) and stimulated with IL-6 for 30 min. Phosphorylation and expression levels of STAT3 and FLAG-PTPs were subjected to immunoblotting analysis using specific antibodies. (C) Recombinant DUSP3 WT or CS was mixed with the p-STAT3 purified from HEK 293 cells and incubated for 1 h at 30°C. Phosphorylation and expression levels of each protein were subjected to immunoblotting analysis using specific antibodies. Non-specific phosphatase CIAP was used as positive control. CS, C124S; CIAP, calf intestinal alkaline phosphatase.