Fig. 1. Optochemical control of protein spatial localization in cell-like compartments.
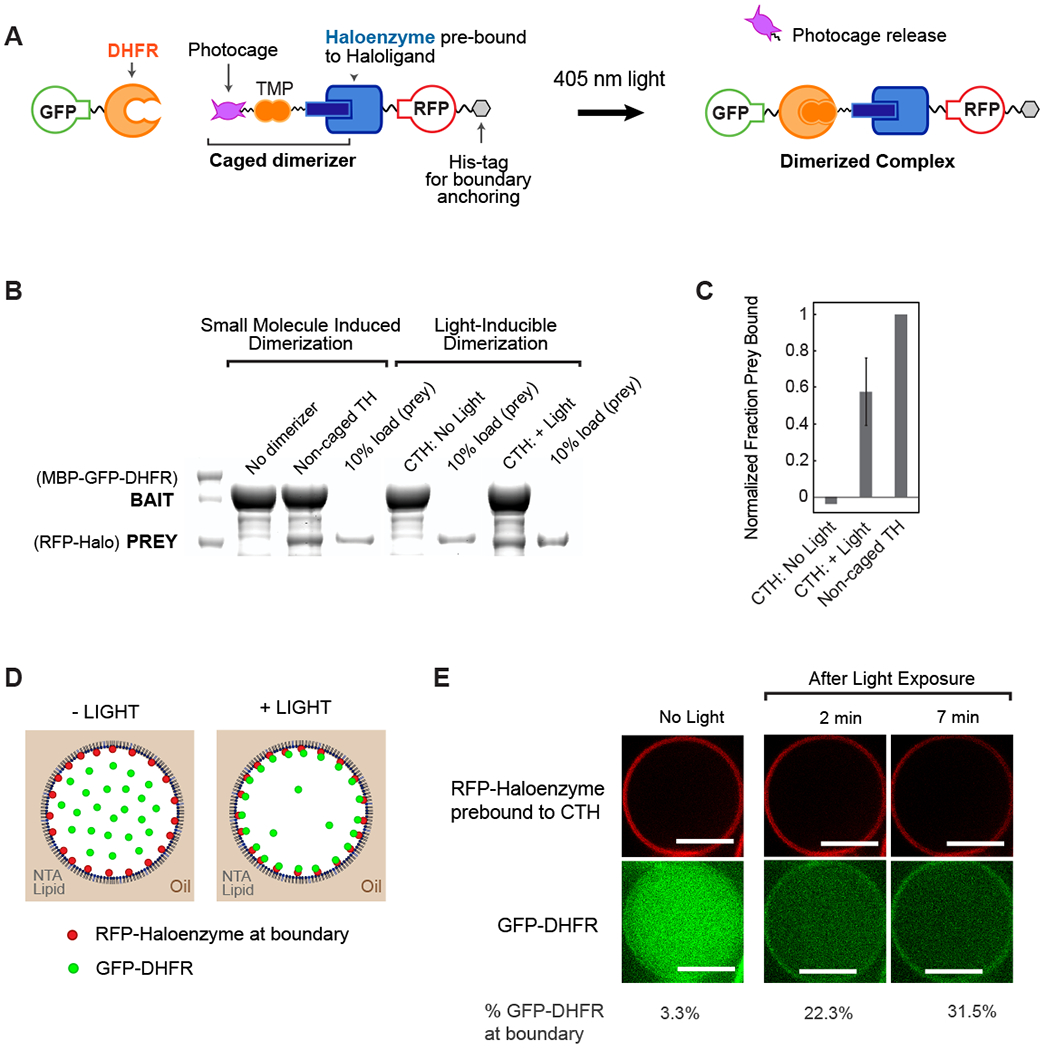
(A) Schematic overview of photocaged TMP-Halo inducible dimerization system. (B) Haloenzyme and DHFR bind to one another in the presence of non-caged TMP-Halo dimerizer, but not in absence of dimerizer, in a pulldown assay. Photouncaging of caged TMP-Halo (CTH) leads to comparable binding to TH; there is undetectable binding in the dark. Caged and non-caged compounds were prebound to Haloenzyme protein. (C) Quantification of CTH and non-caged TH pulldown binding, normalized to positive control, non-caged TH binding. Error bar shows standard deviation from the mean, n = 3 (D) Schematic of light-inducible protein recruitment to the boundary inside synthetic cell-like compartments. 5% DGS-NTA(Ni) lipid and 95% POPC in decane phase. 1 μM His10-RFP-Halo, 0.1 μM GST-GFP-DHFR in aqueous phase. (E) His10-RFP-Halo binds to DGS-NTA(Ni) lipid in the droplet boundary. Recruitment of GST-GfP-DHFR to the boundary is triggered by 405 nM illumination, which uncages CTH. Scalebar 10 μm.
