Abstract
Background
medication-related problems occur frequently when older patients are discharged from hospital. Interventions to support medication use have been developed; however, their effectiveness in older populations are unknown. This review evaluates interventions that support successful transitions of care through enhanced medication continuity.
Methods
a database search for randomised controlled trials was conducted. Selection criteria included mean participant age of 65 years and older, intervention delivered during hospital stay or following recent discharge and including activities that support medication continuity. Primary outcome of interest was hospital readmission. Secondary outcomes related to the safe use of medication and quality of life. Outcomes were pooled by random-effects meta-analysis where possible.
Results
twenty-four studies (total participants = 17,664) describing activities delivered at multiple time points were included. Interventions that bridged the transition for up to 90 days were more likely to support successful transitions. The meta-analysis, stratified by intervention component, demonstrated that self-management activities (RR 0.81 [0.74, 0.89]), telephone follow-up (RR 0.84 [0.73, 0.97]) and medication reconciliation (RR 0.88 [0.81, 0.96]) were statistically associated with reduced hospital readmissions.
Conclusion
our results suggest that interventions that best support older patients’ medication continuity are those that bridge transitions; these also have the greatest impact on reducing hospital readmission. Interventions that included self-management, telephone follow-up and medication reconciliation activities were most likely to be effective; however, further research needs to identify how to meaningfully engage with patients and caregivers to best support post-discharge medication continuity. Limitations included high subjectivity of intervention coding, study heterogeneity and resource restrictions.
Keywords: medication management, systematic review, continuity of care, hospital discharge, older people
Key points
Medication-related problems occur frequently when older patients are discharged from hospital.
Interventions that best support older patients’ medication continuity are those that bridge transitions.
Interventions that included self-management, telephone follow-up and medication reconciliation activities were most effective.
Introduction
Medication management processes and behaviours support safe and effective medication use. These involve healthcare professionals, caregivers, organisations and the patient themselves. Medication-related problems (MRPs) and interruptions to, or discontinuity of, medication management occur frequently when older patients are discharged from hospital [1–4]. MRPs can lead to hospital readmission and poorer quality of life (QoL), resulting in higher healthcare utilisation [5, 6]. Specific problems include reconciliation errors [7], patient confusion [3], inappropriate continuation of short-term medication [8] and inadequate monitoring [9].
Better and safer care transitions, especially hospital discharge, are an international priority [10–12]. Burke et al.’s ideal transition-of-care framework [12] recognises medication safety as a crucial element for successful transitions. Evaluation of interventions to support medication continuity indicated that patient education at discharge reduced the risk of adverse medication-related events, although evidence remains limited [13]. An American study further highlighted the value of pharmacy-supported interventions in reducing hospital readmissions [14]. However, neither of these studies evaluated the effectiveness of interventions delivered specifically to older populations.
Other systematic reviews have identified discharge interventions that reduce negative patient outcomes; however, their focus was broader than medications [15, 16]. Evaluation of complex interventions, defined as those involving multiple components, outcomes, target behaviours or flexibility [17] is notoriously difficult [18, 19]. To address this, Leppin and colleagues (building on work of Hansen et al. [16]) developed a taxonomy of interventional components allowing in-depth comparison and meta-analysis [15]. Guidance published in 2000 by the UK’s Medical Research Council (MRC), who invest in research, also established an influential good practice framework [17] to help overcome evaluation challenges.
This review aims to build on this previous knowledge by evaluating interventions, aimed at supporting successful transitions of care for older patients through enhanced medication continuity, using a taxonomy of components.
Methods
To promote rigour and transparency, the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist is presented (Supplementary Material A1) and the review is registered [PROSPERO (CRD42018086873)].
Search strategy
Published studies from 1st January 2003 to 1st September 2019 were sought from electronic databases (MEDLINE, EMBASE, CINAHL, PsycINFO, clinicaltrials.gov and Cochrane Database of Systematic Reviews). The start date of 2003 was chosen to coincide with predicted uptake of MRC guidance by researchers, as demonstrated by Datta and Petticrew [18], and therefore its subsequent implementation within trials.
Medical Subject Headings chosen in collaboration with a subject librarian, including key search terms related to care transitions (e.g. transitional care, patient handoff and discharge), were combined with those related to medication continuity (e.g. pharmacy services, medication systems and safety) (see Supplementary Material A2). Additional citations were identified through hand-searching reference lists and forward citation search. English language restrictions were imposed due to time and resource limitations.
Selection criteria
Inclusion and exclusion criteria
Eligible studies included participants with a mean age of 65 years or older, who were being prepared for hospital discharge or who had a recent discharge (intervention provided within 1 month of discharge or on first post-discharge primary care visit). Study interventions had to describe activities relating to medication that supported continuity. Outcomes of successful transitions were of interest; primarily a reduction in hospital readmission rates. Secondary outcomes relating to the safe use of medication (e.g. MRPs and discrepancies) and QoL were also included as these factors contribute to successful transitions and can be mediated through medication continuity. The search was limited to randomised controlled trials (RCT) and cluster RCT (cRCT) as these are considered the gold standard in the hierarchy of evidence [20].
Selection process
One reviewer (JT) independently screened titles and abstracts against the selection criteria, removing duplicates. Those rejected were reviewed by a second author (VC) to reduce the exclusion of potentially relevant publications [21]. Disagreements were discussed and final inclusion was determined after full-text review.
Data extraction and quality assessment
Data extraction was performed independently by two reviewers (JT and VC) using a predefined template. Abstracted data included demographics, intervention details, outcome measures and findings. Protocols or further detail from the study authors were sought wherever possible.
The methodological risk of bias was independently assessed in accordance with the Cochrane Handbook [22] and the guidelines of the Cochrane Consumers and Communication Review Group [23]. Five domains were rated: random sequence generation, allocation sequence concealment, blinding (outcome assessment), completeness of outcome data and selective reporting. Performance bias was not assessed because blinding of participants and intervention personnel would be impossible.
Data synthesis and analysis
Information was used to form a description of the intervention components each patient received (when, how often and for how long). These activities were coded independently (JT and VC), guided by an adapted version of Leppin et al.’s taxonomy of interventional activities [15], modified by the reviewers for medication-related activities (see Table 1). Disagreements were resolved through discussion. Meta-analysis of all-cause readmission data was performed (where the risk ratio (RR) and 95% confidence intervals (CIs) could be calculated) using the longest reported follow-up period. Outcome effects were pooled using a Mantel–Haenszel random-effects model in Cochrane Review Manager (RevMan) V5.3 software. The I2 statistic was calculated to describe the percentage of variation due to heterogeneity rather than chance and publication bias was assessed. No other outcome data could be pooled due to variance in reporting measures.
Table 1.
Taxonomy of discharge interventions [4] adapted by the reviewers for medication continuity
| Medication-related activity component | Description |
|---|---|
| Follow-up | |
| Telephone | Use of a telephone or videophone for provider-initiated communication after discharge that does not occur in the control arm |
| Home visit | Physical visit by intervention provider to patient’s place of residence when this does not happen in the control arm |
| Patient education | Patient-directed education related to medication but not focused on encouraging self-management and not occurring in the control arm |
| Self-management (education or coaching) | Patient-directed education or coaching directly focused on improving the patient’s ability to self-manage their medication needs that does not happen in the control arm |
| Medication intervention: reconciliation | Creating the most accurate list possible of all medications a patient is taking and comparing it to the current order, with the goal of providing correct medications at all transition points when this does not happen or is performed by usual care staff in the control arm |
| Medication intervention: review | Critical examination of a patient’s medication with the objective of reaching an agreement with the patient about treatment optimisation when this does not happen in the control arm |
| Patient-centred discharge document | Some difference in the format or usability of discharge materials to make them more relevant or accessible when compared to the control arm |
| Collaboration within care team | Healthcare professionals cooperatively working together, sharing responsibility for problem-solving and making decisions to carry out medication-related plans for patient care |
| Timely cross-sector communication | Engagement with other sector provider in communication about patient medication status when this does not occur or occurs at a later date in the control arm |
| Patient hotline | Presence of an open line for patient-initiated communication when this either does not exist in the control arm or is more restricted in availability or usefulness |
Results
Study inclusion
The search identified 2394 unique citations. A total of 2278 were excluded following title and abstract review. Full-text publications were assessed for 116 studies, resulting in 24 that met the selection criteria (see Figure 1). Consensus between reviewers was 94% with no studies excluded.
Figure 1.
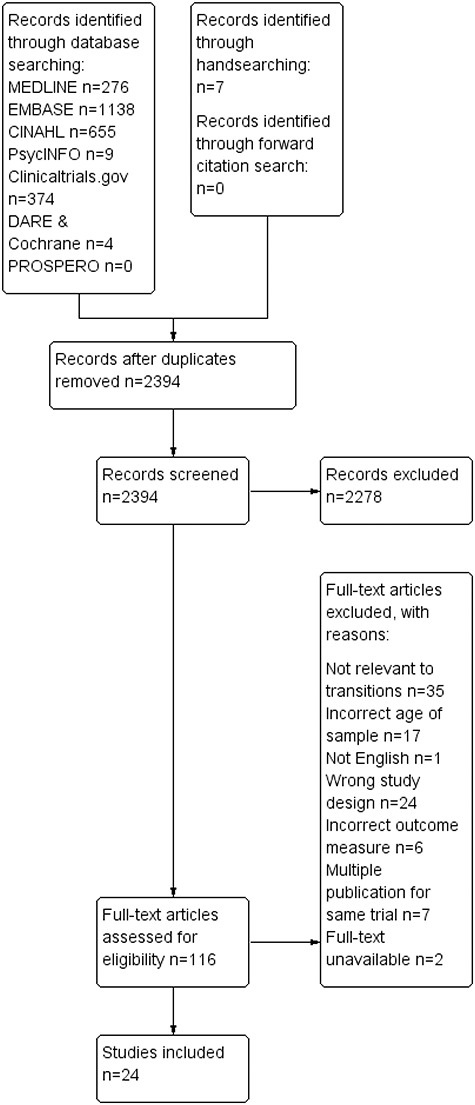
PRISMA flow diagram of literature search and included studies.
Study characteristics
Studies were conducted in 12 countries covering a range of public and privately funded healthcare systems (see Figure 2 for summary of characteristics). A total of 17,664 participants were enrolled (range, 25 [24]–4656 participants [25]) and the sample’s mean age ranged from 66 [26, 27] to 86 years [28] (Supplementary Material A3 provides full study characteristics).
Figure 2.
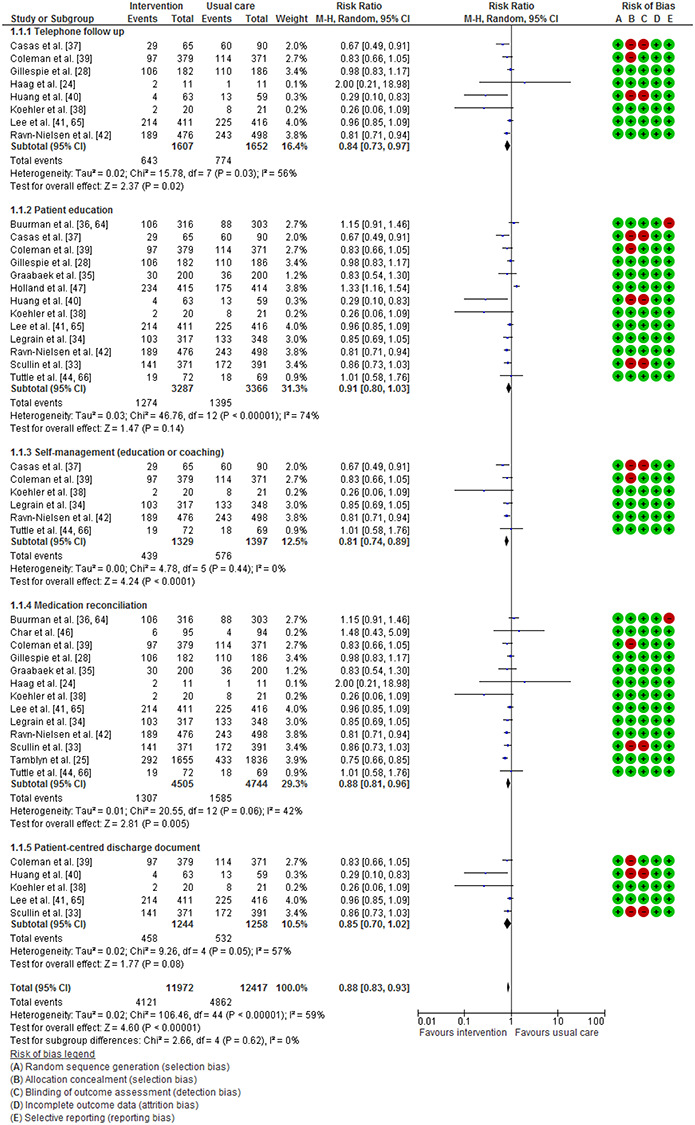
Effect of intervention activity component compared with usual care on all-cause hospital readmission (a summary of five activity components).
Nine studies described intervention bundles provided during hospital admission [25, 27, 29–35], seven of which were delivered by the inpatient pharmacy team and one by geriatricians [34]. One involved an electronic intervention [25]. Intervention components were most often delivered once during the inpatient stay. Nine interventions were commenced during admission and continued post-discharge, bridging the transition [26, 28, 36–42]. Five of these involved nurse-delivered interventions, sometimes acting as ‘transition coaches’, to facilitate the patient’s role in self-care. Three were pharmacist-led [28, 38, 42] and one was multidisciplinary [41]. A further six studies evaluated interventions that commenced post-discharge [24, 43–47], of which five were delivered by pharmacists. One study [45] involved automatic electronic transfer of patient information to the primary care provider. Overall, intervention delivery ranged from a single time point to 12 months post-discharge. The most intensive activity period was between discharge and 3 months post-discharge.
Risk of bias
Twelve studies scored low in all five risks of bias domains [24, 25, 28, 34, 35, 38, 41, 42, 44–47] (see Supplementary Material A4). Nine studies were rated as having the highest risk of bias [26, 27, 29, 31, 33, 37, 39, 40, 43] based on randomisation and allocation concealment methods. High risk of bias was found mainly in blinding of outcome assessments (n = 7 studies).
Intervention component characteristics
Supplementary Material A5 summarises the medication-related activity components coded within each study using the adapted taxonomy. Inter-rater agreement was high (k = 0.77).
Studies used varying numbers and combinations of activities within intervention bundles. Most studies utilised three or more activities (mean = 4.6; range 1–8). Three studies involved single-component interventions [27, 30, 45]. The range of time to first post-discharge activity was 2 days to 2 months.
Interventions offered during hospital admission
Table 2 shows that the most commonly reported activities were patient education (n = 5; 56%), reconciliation (n = 6; 67%), provision of patient-centred documentation (n = 4; 66%) and timely cross-sector communication (n = 7; 78%). Two studies showed a reduction in hospital readmissions [33, 34] whilst Basger et al. [29] demonstrated a statistically significant difference within the vitality domain of the Health-related QoL measure. All three of these interventions included medication review and reconciliation, patient education and transfer of information; however, only Legrain et al.’s study [34] was considered to be at low risk of bias. Discrepancies in medication were improved by Hockly et al.’s intervention involving transfer of discharge information (P = 0.00034) [27], Tamblyn et al.’s medication reconciliation intervention (odds ratio [OR] 0.24; CIs 0.12–0.57) [25] and Bolas et al.’s intervention (P < 0.005) [31] involving education and a personalised medication sheet. Only Tamblyn et al.’s study [25] was considered to be high quality, with the other studies having selection or detection biases.
Table 2.
Summary of study characteristics
| Study | Participants (I= intervention arm; C= control arm) | Intervention components coded using taxonomy | Provider | Control | Outcome measure | |
|---|---|---|---|---|---|---|
| Age = years to nearest whole | Result | Overall effect | ||||
| Interventions offered during hospital admission | ||||||
| Basger et al. [29] | n = 216 | E/S/MR/CR/CSC | Pharmacist | Usual care | MRP detection between follow-up and discharge; (0.09 ≤ P ≤ 0.97) | No difference |
| I: 114 | QoL; I: +18.6 versus C: +15.3 (P < 0.001) | Favours intervention | ||||
| C: 102 | ||||||
| Mean age = 81 | ||||||
| Bolas et al. [31] | n = 162 | E/MR/D/CSC/H | Pharmacist | Usual care | Readmissions (3 month); figures NR (P > 0.05) | No difference |
| I: 81 | Error (drug name); I: 1.5% versus C: 7% (P < 0.005) | Favours intervention | ||||
| C: 81 | Error (drug dose); I: 10% versus C: 17% (P < 0.07) | No difference | ||||
| Mean age = 74 | Error (dose frequency); I: 11% versus C: 18% (P < 0.004) | Favours intervention | ||||
| Graabaek et al. [35] | n = 400 | E/MR/CR/C | Pharmacist | Usual care | Readmissions (1 month); I: 30 (15%) versus C: 36 (18%) (P = 0.72) | No difference |
| I: 200 | Mortality (3 month); I: 13 (6.5%) versus C: 16 (8%) (P = 0.6) | No difference | ||||
| C: 200 | ||||||
| Mean age = 75 | ||||||
| Hockley et al. [27] | n = 33I: 17 | CSC | Pharmacist | Usual care | Incidence of discrepancy (GP data); I: 25 (14%) versus C: 50 (26%) (P = 0.00034) | Favours intervention |
| C: 16Mean age = 66 | Incidence of discrepancy (patient reported); I: 10 (8%) versus C: 31 (23%) (P = 0.000043) | Favours intervention | ||||
| Lalonde et al. [32] | n = 83 | D/C/CSC | Pharmacist | Usual care | Error rate; I: 13.2% versus C: 15.3% (P = 0.6) | No difference |
| I: 42 | ||||||
| C: 41 | ||||||
| Mean age = 71 | ||||||
| Legrain et al. [34] | n = 665 | E/S/MR/CR/C/CSC | Geriatrician | Usual care | Readmission (3 month); I: 64 (20.2%) versus C: 99 (28.4%) (P = 0.01) | Favours intervention |
| I: 317 | Readmission (6 month); I: 103 (32.5%) versus C: 133 (38%) (P = 0.12) | No difference | ||||
| C: 348 | Mortality (6 month); I: 56 (17.7%) versus C: 65 (18.7%) (P = 0.74) | No difference | ||||
| Mean age = 85 | ||||||
| Scullin et al. [33] | n = 762 | E/MR/CR/D/CSC | Pharmacist and pharmacy technician | Usual care | Readmission (12 month); I: 141 (38%) versus C: 172 (44%) (P = 0.027) | Favours intervention |
| I: 371 | ||||||
| C: 391 | ||||||
| Mean age = 70 | ||||||
| Tamblyn et al. [25] | n = 4656 | MR/CSC | Electronic intervention | Usual care | Adverse drug event; I: 76 (4.6%) versus C: 73 (4%) (OR 0.24; CIs 0.33–1.48) | No difference |
| I: 2203 | Medication discrepancy; I: 437 (26.4%) versus C: 1029 (56%) (OR 0.24; CIs 0.12–0.57) | Favours intervention | ||||
| C: 2453 | Readmission (1 month); I: 170 (10.3%) versus C: 261 (14.2) (OR 0.22; CIs 0.06–1.14) | No difference | ||||
| Mean age = 70 | Readmission (3 month); I: 292 (17.6%) versus C: 433 (23.6%) (OR 0.37; CIs 0.11–1.40) | No difference | ||||
| Tong et al. [30] | n = 832 | D | Pharmacist | Usual care | Error rate; I: 15% versus C: 61.5% (P < 0.01) | Favours intervention |
| I: 401 | ||||||
| C: 431 | ||||||
| Mean age = 73 | ||||||
| Interventions commenced during hospital admission that include continuing support post-discharge | ||||||
| Buurman et al. [36, 64] | n = 674 | V/E/MR/C/CSC | Nurse | Usual care | Readmission (6 month); I: 106 (33.5%) versus C: 88 (29%) (P = NR) | No difference |
| I: 337 | Mortality (6 month); I: 85 (25.2%) versus C: 104 (30.9%) (P = 0.045) | Favours intervention | ||||
| C: 337 | ||||||
| Mean age = 80 | ||||||
| Casas et al. [37] | n = 155 | T/E/S/CSC | Nurse | Usual care | Readmission (12 month); I: 29 (45%) versus C: 60 (67%) (P = 0.028) | Favours intervention |
| I: 65 | Mortality (12 month); I: 12 (19%) versus C: 14 (16%) (P = 0.67) | No difference | ||||
| C: 90 | ||||||
| Mean age = 71 | ||||||
| Chan et al. [26] | n = 699 | T/E/S/MR/D/H | Nurse | Usual care | Care Transitions Measure-3 score; I: 80.5% versus C: 78.5% (P = 0.18) | No difference |
| I: 347 | ||||||
| C: 353 | ||||||
| Mean age = 66 | ||||||
| Coleman et al. [39] | n = 750 | T/V/E/S/MR/D | Nurse | Usual care | Readmission (1 month); I: 31 (8.3%) versus C: 44 (11.9%) (P = 0.048) | Favours intervention |
| I: 379 | Readmission (3 month); I: 63 (16.7%) versus C: 83 (22.5%) (P = 0.04) | Favours intervention | ||||
| C: 371 | Readmission (6 month); I: 97 (25.6%) versus C: 114 (30.7%) (P = 0.28) | No difference | ||||
| Mean age = 76 | ||||||
| Gillespie et al. [28] | n = 400I: 199C: 201Mean age = 86 | T/E/MR/CR/C/CSC | Pharmacist | Usual care | Readmission (12 month); I: 106 (58.2%) versus C: 110 (59.1%) (OR 0.96; CIs 0.64–1.46)Drug related readmission; I: 9 (4.9%) versus C: 45 (24%) (OR 0.20; CIs 0.10–0.41) | No differenceFavours intervention |
| Huang et al. [40] | n = 126 | T/V/E/D/C/CSC/H | Nurse | Usual care | Readmission (3 month); I: 4 (6.35%) versus C: 13 (20.6%) (P = 0.02) | Favours intervention |
| I: 63 | QoL; I: +18.6 versus C: +15.3 (P < 0.001) | Favours intervention | ||||
| C: 63 | ||||||
| Mean age = 77 | ||||||
| Koehler et al. [38] | n = 41I: 20C: 21 | T/E/S/MR/CR/D/CSC | Nurse and pharmacist | Usual care | Readmission (1 month); I: 2 (10%) versus C: 8 (38%) (P = 0.03)Readmission (2 month); I: 4 (20%) versus C: 1 (5%) (P = 0.18) | Favours interventionNo difference |
| Mean age = 79 | ||||||
| Lee et al. [41, 65] | n = 840I: 420C: 420Mean age = 69 | T/V/E/MR/CR/D/C/CSC | Multidisciplinary team | Usual care | Readmission (1 month); I: 117 (28.5%) versus C: 139 (33%) (P = 0.124)Readmission (2 month); I: 183 (44.5%) versus C: 186 (44%) (P = 0.957)Readmission (3 month); I: 214 (52%) versus C: 225 (54%) (P = 0.561)Mortality; HR 0.72; CIs 0.61–0.86; P < 0.001 | No differenceNo differenceNo differenceFavours intervention |
| Ravn-Nielsen et al. [42] | n = 974I: 476C: 498Mean age = NR | T/E/S/MR/CR/C/CSC | Pharmacist | Usual care | Readmission (1 month); I: 68 (14.3%) versus C: 111 (22.3%) (HR 0.62; CIs 0.46–0.84)Readmission (6 month); I: 189 (39.7%) versus C: 243 (48.8%) (HR; 0.75 CIs 0.62–0.9)Mortality (6 month); I: 54 (11.3%) versus C: 50 (10%) (HR 1.05; CIs 0.68–1.63) | Favours interventionFavours interventionNo difference |
| Interventions commenced post-discharge | ||||||
| Ahmad et al. [43, 48] | n = 340I: 180C: 160Mean age = 70 | V/E/MR/CR/C | Pharmacist and pharmacy technician | Usual care | MRPs: (mean number of problems) I: 1.51 baseline to 1.37 follow up versus C: 1.58 to 1.62 (P = NR) | No difference |
| Char et al. [46] | n = 200I: 100C: 100Mean age = 74 | MR/C | Pharmacist | Usual care | Errors: (number) I: 15 (15.8%) versus C: 54 (57.4%) (P < 0.001)Readmission (1 month); I: 6 (6%) versus C: 4 (4%) (P = NR) | Favours interventionNo difference |
| Gurwitz et al. [45] | n = 3661I: 1870C: 1791Mean age = NR | CSC | Automated electronic system | Usual care | Readmission (1 month); I: 351 (18.8%) versus C: 356 (19.9%) (HR 0.94; CIs 0.91–1.1) | No difference |
| Haag et al. [24] | n = 25I: 13C: 12Mean age = 84 | T/MR/CR/C | Pharmacist | Usual care | Readmission (1 month); I: 2 (18%) versus C: 1 (9%) (P = 0.53)Medication appropriateness1 (30 day); I: 10 (91%) versus C: 10 (91%) (P > 0.99)Medication appropriateness 2 (30 day); I: 8 (73%) versus C: 10 (91%) (P = 0.31)Medication appropriateness 3 (30 day); I: 9 (82%) versus C: 10 (91%) (P = 0.55) | No differenceNo differenceNo differenceNo difference |
| Holland et al. [47] | n = 872I: 437C: 435Mean age = 85 | V/E/CR/C/CSC | Pharmacist | Usual care | Readmission (6 month); I: 234 (54.5%) versus C: 178 (41.8%) (P = 0.009)QoL; I: −0.131 versus C: −0.137 (P = 0.84)Visual analogue scale I: −7.36 versus C: −3.24 (P = 0.042) | Favours controlNo differenceFavours control |
| Tuttle et al. [44, 66] | n = 159 | V/E/S/MR/CR/C | Pharmacist | Usual care | Readmission (3 month); I: 19 (26%) versus C: 18 (26%) (P = 0.95) | No difference |
| I: 84 | ||||||
| C: 75 | ||||||
| Mean age = 69 | ||||||
C, collaboration within care team; CIs, confidence intervals; CR, clinical review; CSC, timely cross-sector communication; D, patient-centred discharge document; E, education; H, home visit; HR, hazard ratio; MR, medication reconciliation; MRP, Medication-related problem; NR, not reported; QoL, quality of life; S, self-management (education or coaching); T, telephone follow-up; V, home visit.
Interventions commenced during hospital admission and include continuing support post-discharge
The most widely used activity was patient education (n = 9; 100%) (see Table 2). Three studies provided education once, Casas et al. provided a two hour educational programme at discharge [37], Huang et al. a medication safety information brochure [40] and Ravn-Nielsen et al. used a 30-min motivational interview [42]. Two studies utilised ‘transition coaches’ to deliver education throughout follow-up [36, 39]. Three studies provided education at admission and discharge using pharmacists (to advise on medication changes) or nurses (to advise on chronic conditions) [28, 38, 41]. One study [26] provided disease-specific education in the participant’s native language. Medication reconciliation (n = 7; 78%) and patient-centred discharge documentation (n = 5; 63%), such as a ‘personal health record’ containing medication information [39], were also used. Post-discharge telephone calls (n = 5) to provide reinforcement of self-management [37, 39], further education [26, 38, 40, 42] and assessment of adherence [28, 41], were conducted more frequently than home visits (n = 1). Three studies [39–41] used both methods, conducting a home visit within the first week post-discharge and subsequent weekly telephone calls.
Five of these studies demonstrated a statistically significant reduction in all-cause hospital readmissions [37–40, 42]. All five interventions included follow-up (telephone, home visit or both) and education, continuing until seven [38] to 180 days post-discharge [42]. Four of these studies [26, 37, 39, 40] were considered to be at the highest risk of bias, however, as allocation was not concealed or outcome assessors were not blinded. Chan et al. [26] did not find any difference between arms with the Care Transitions Measure-3 score, which assesses the quality of the transitional care experience (P = 0.18); however, Huang et al. [40] found a greater improvement in QoL score within their intervention arm (I: +18.6 versus C: +15.3; P < 0.001).
Interventions commenced post-discharge
The majority of post-discharge interventions were provided by pharmacy staff (n = 5): community pharmacists [43]; outpatient polyclinic pharmacists [46]; and trained intervention pharmacists [24, 44, 47]. Table 2 shows that medication reconciliation and medication review were provided in most of the intervention bundles (n = 4; 66%). Home visits (n = 4) were conducted more frequently than telephone calls (n = 1).
Of the six interventions, none showed a statistically significant reduction in hospital readmission and all were considered to be high quality. Holland et al. demonstrated a 30% increase in readmission rates (P = 0.009) in their intervention arm [47], involving review and education, and a decrease in visual analogue QoL scores (I: −7.36 versus C: −3.24; P = 0.042). Other studies reported a reduction in MRPs [48] (not statistically significant) and improvement in medication discrepancies (P < 0.001) [46] by using pharmacists for post-discharge review or reconciliation.
Meta-analysis
Nineteen studies reported hospital readmission data and were therefore combined using meta-analysis (Figure 2) (see Supplementary Material A6 for full forest plot). One could not be included [31] as the results were reported in a way that did not allow calculation of RR. Significant variability across studies was observed (I2 = 70%). The meta-analysis, stratified by component, demonstrated that the activities associated with reduced hospital readmissions were self-management education or coaching (RR 0.81 [0.74, 0.89]), telephone follow-up (RR 0.84 [0.73, 0.97]) and medication reconciliation (RR 0.88 [0.81, 0.96]). Other components that were close to statistical significance were patient-centred discharge documents (RR 0.85 [0.70, 1.02]) and education (RR 0.91 [0.80, 1.03]. There was no evidence of publication bias (see Supplementary Material A7).
Discussion
This systematic review aimed to evaluate the evidence for interventions that support successful transitions of care for older people through enhanced medication continuity. We found interventions that bridged the transition for up to 90 days were more likely to support successful transitions and reduce adverse outcomes. These interventions used on average more components than those focusing solely on hospital admission or post-discharge time periods (6.2 versus 3.6 versus 3.8 respectively), reflecting their higher intensity and longitudinal nature. Other reviews of discharge interventions have shown that multiple components are significantly more effective than a single activity [16, 49–51] and that their effects are sustained [49]. Actual time taken to deliver the intervention components was rarely reported, but is important to consider in the context of busy healthcare settings. For example, Ravn-Nielsen et al. [42] reported an average of 114 min spent per patient. The longer term sustainability of resource intensive interventions such as these and how they can be integrated into ‘usual care’ should be deliberated.
In this review, patient education, reconciliation and timely cross-sector communication were the most widely used activities. Reconciliation, performed manually or via electronic intervention, was shown to significantly reduce hospital readmission (RR 0.88 [0.81, 0.96]) and was linked to fewer medication errors [25, 29, 31, 48]. The benefits of reconciliation appear highly contested in the literature. When provided after hospital discharge, reconciliation has not been shown to effectively reduce post-discharge harm or improve health outcomes [52]. However, reconciliation provided during admission has demonstrated a reduction in healthcare utilisation and improved patient safety [53, 54].
Interventions in this review were delivered by a range of healthcare professionals, with no professional appearing more effective than the other. Ten studies [33, 34, 37, 40–44, 46, 47] also involved caregivers; mostly as an information source during reconciliation activities. Caregivers often support older patients during their day-to-day health management and can effectively promote self-management [55]. They could, therefore, be engaged in wider activities amongst these interventions and further work should identify opportunities for caregiver involvement within medication continuity.
The most effective component within these intervention bundles was self-management coaching or education. Promoting self-management in older patients has received global attention as it is thought to improve a patient’s ability to manage their long-term conditions. Despite this, self-management activities were used in less than half of included studies (n = 8). It is known that older people with low levels of social, cognitive, and physical functioning are generally poorer self-managers [56]. Therefore, how such individuals are supported to self-manage their medication through interventions such as these requires further attention.
Telephone follow-up (RR 0.84 [0.73, 0.97]) also reached statistical significance within our meta-analysis. Other reviews of telephone follow-up interventions [57–59] have been unable to demonstrate a reduction in readmission rates; however Crocker et al. [57] highlighted that patient engagement with post-discharge clinical contact was improved. This contact may, therefore, provide opportunities for reinforcement of educational messages and resolution of MRPs; however, barriers to implementation (e.g. time, cost and personnel resourcing) may limit its use. Patient-centred health documentation has practical and psychological benefits for patients, such as bolstering memory, as a tool for sharing information or feeling more empowered to ask health-related questions [60]. Within our review, it is unclear how patients made use of their personalised documentation; however, all examples included an up-to-date list of their medications presented in an acceptable format.
There is consensus that timely cross-sector communication supports medication continuity at transitions [50, 61]. Although much emphasis has been given to improving communication at transitions [62], our meta-analysis did not find a significant effect on readmission rates (RR 0.90 [0.79, 1.02]). There have been technological advances to support timely communication and many of the included studies transferred information to the primary care provider, community pharmacy or outpatient services at discharge. Specific methods included as follows: fax [25, 27, 31–33, 38], telephone [34, 42], email [26] and secure electronic platform [24, 37, 45]. We found no interventions describing a method allowing primary care providers to readily communicate back to hospital providers. This is a barrier to medication continuity within the UK primary care sector when clarification or further information is required [61]. Further studies are needed to test interventions supporting this aspect of cross-sector communication.
Limitations
Studies were highly heterogeneous, drawn from varying populations, care settings and included different combinations of components and delivery time points. It is difficult to attribute success to individual components within bundles and our meta-analysis illustrates a modest overall effect size. Therefore, these results cannot demonstrate causality and we cannot draw firm conclusions. There is currently no validated medication continuity-related measure, which would have allowed us to better combine results. Three potential studies were also excluded due to English language restrictions and unavailability of full-texts.
Coding intervention components can be a highly subjective process [63]. We used our best judgement, especially when intervention descriptions were lacking detail. To reduce bias, two reviewers independently coded the components. Interventions were only coded if the activity was explicitly stated.
Most of the included studies contained methodological flaws, which affected their risk of bias assessment. It was unclear whether appropriate methods were in fact utilised and not reported or simply not performed at all. To improve future trials, studies must ensure absolute blinding of outcome assessors and that allocation concealment and randomisation are appropriately performed and documented.
Conclusion
Overall, our results suggest that interventions that bridge the care transition best support older patients’ medication continuity and have the greatest impact on reducing hospital readmission. Interventions that included self-management, telephone follow-up and medication reconciliation activities were most likely to be effective. Further work needs to identify how best to engage with patients and their caregivers in order to better support post-discharge medication continuity.
Supplementary Material
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This manuscript presents independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant PB-PG-0317-20010). The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. This work was supported by the NIHR Yorkshire and Humber Patient Safety Translational Research Centre.
(As per Author Guidelines, for the full reference list, please refer to Supplementary Material A8)
References
- 6. Parekh N, Ali K, Stevenson JM et al. . Incidence and cost of medication harm in older adults following hospital discharge: a multicentre prospective study in the UK. Br J Clin Pharmacol 2018; 84: 1789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spinewine A, Claeys C, Foulon V, Chevalier P. Approaches for improving continuity of care in medication management: a systematic review. International J Qual Health Care 2013; 25: 403. [DOI] [PubMed] [Google Scholar]
- 14. Rodrigues CR, Harrington AR, Murdock N et al. . Effect of pharmacy-supported transition-of-care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother 2017; 51: 866–89. [DOI] [PubMed] [Google Scholar]
- 15. Leppin AL, Gionfriddo MR, Kessler M et al. . Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med 2014; 174: 1095–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008; 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haag JD, Davis AZ, Hoel RW et al. . Impact of pharmacist-provided medication therapy management on healthcare quality and utilization in recently discharged elderly patients. Am Health Drug Benefits 2016; 9: 259–67. [PMC free article] [PubMed] [Google Scholar]
- 25. Tamblyn R, Abrahamowicz M, Buckeridge DL et al. . Effect of an electronic medication reconciliation intervention on adverse drug events: a cluster randomized trial. JAMA Netw Open 2019; 2: e1910756-e. doi: 10.1001/jamanetworkopen.2019.10756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan B, Goldman LE, Sarkar U et al. . The effect of a care transition intervention on the patient experience of older multi-lingual adults in the safety net: Results of a randomized controlled trial. J Gen Intern Med 2015; 30: 1788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hockly M, Williams S, Allen M. Transfer of care – a randomised control trial investigating the effect of sending the details of patients' discharge medication to their community pharmacist on discharge from hospital. Int J Pharm Pract 2018; 26: 174–82. [DOI] [PubMed] [Google Scholar]
- 28. Gillespie U, Alassaad A, Henrohn D et al. . A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med 2009; 169: 894–900. [DOI] [PubMed] [Google Scholar]
- 29. Basger BJ, Moles RJ, Chen TF. Impact of an enhanced pharmacy discharge service on prescribing appropriateness criteria: A randomised controlled trial. Int J Clin Pharm 2015; 37: 1194–205. [DOI] [PubMed] [Google Scholar]
- 30. Tong EY, Roman CP, Mitra B et al. . Reducing medication errors in hospital discharge summaries: A randomised controlled trial. Med J Aust 2017; 206: 36–9. [DOI] [PubMed] [Google Scholar]
- 31. Bolas H, Brookes K, Scott M, McElnay J. Evaluation of a hospital-based community liaison pharmacy service in Northern Ireland. Pharm World Sci 2004; 26: 114–20. [DOI] [PubMed] [Google Scholar]
- 32. Lalonde L, Lampron A, Vanier M, Levasseur P, Khaddag R, Chaar N. Effectiveness of a medication discharge plan for transitions of care from hospital to outpatient settings. Am J Health Syst Pharm 2008; 65: 1451–7. [DOI] [PubMed] [Google Scholar]
- 33. Scullin C, Scott MG, Hogg A, McElnay JC. An innovative approach to integrated medicines management. J Eval Clin Pract 2007; 13: 781–8. [DOI] [PubMed] [Google Scholar]
- 34. Legrain S, Tubach F, Bonnet-Zamponi D et al. . A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: The optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc 2011; 59: 2017–28. [DOI] [PubMed] [Google Scholar]
- 35. Graabæk T, Hedegaard U, Christensen MB, Clemmensen MH, Knudsen T, Aagaard L. Effect of a medicines management model on medication-related readmissions in older patients admitted to a medical acute admission unit—a randomized controlled trial. J Eval Clin Pract 2019; 25: 88–96. [DOI] [PubMed] [Google Scholar]
- 36. Buurman BM, Parlevliet JL, van BAJ, de RJ, de SE. A randomised clinical trial on a comprehensive geriatric assessment and intensive home follow-up after hospital discharge: the transitional care bridge. BMC Health Serv Res 2010; 10: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casas A, Troosters T, Garcia-Aymerich J et al. . Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J 2006; 28: 123–30. [DOI] [PubMed] [Google Scholar]
- 38. Koehler BE, Richter KM, Youngblood L et al. . Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med 2009; 4: 211–8. [DOI] [PubMed] [Google Scholar]
- 39. Coleman EA, Parry C, Chalmers S, Min S. The care transitions intervention: Results of a randomized controlled trial. Arch Intern Med 2006; 166: 1822–8. [DOI] [PubMed] [Google Scholar]
- 40. Huang T-T, Liang S-H. A randomized clinical trial of the effectiveness of a discharge planning intervention in hospitalized elders with hip fracture due to falling. J Clin Nurs 2005; 14: 1193–201. [DOI] [PubMed] [Google Scholar]
- 41. Lee KH, Low LL, Allen J et al. . Transitional care for the highest risk patients: findings of a randomised control study. Int J Integr Care 2015; 15: e039. doi: 10.5334/ijic.2003 2015; 15,e039-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ravn-Nielsen LV, Duckert M-L, Lund ML et al. . Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Intern Med 2018; 178: 375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahmad A, Hugtenburg J, Welschen LMC, Dekker JM, Nijpels G. Effect of medication review and cognitive behaviour treatment by community pharmacists of patients discharged from the hospital on drug related problems and compliance: design of a randomized controlled trial. BMC Public Health 2010; 10: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alicic RZ, Short RA, Corbett CL et al. . Medication intervention for chronic kidney disease patients transitioning from hospital to home: Study design and baseline characteristics. Am J Nephrol 2016; 44: 122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gurwitz JH, Field TS, Ogarek J et al. . An electronic health record-based intervention to increase follow-up office visits and decrease rehospitalization in older adults. J Am Geriatr Soc 2014; 62: 865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Char CWT, Yip AYF, Kee KW, Lee ES, Chua AHL. Effectiveness of pre-consultation medication reconciliation service in reducing medication discrepancies during transition of care from hospital discharge to primary care setting in Singapore - a randomised controlled trial. J Appl Pharm 2017; 9. [Google Scholar]
- 47. Holland R. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ 2005;330:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahmad A, Nijpels G, Dekker JM, Kostense PJ, Hugtenburg JG. Effect of a pharmacist medication review in elderly patients discharged from the hospital. Arch Intern Med 2012; 172: 1346–7. [DOI] [PubMed] [Google Scholar]
- 56. Cramm JM, Hartgerink JM, Steyerberg EW, Bakker TJ, Mackenbach JP, Nieboer AP. Understanding older patients’ self-management abilities: Functional loss, self-management, and well-being. Qual Life Res 2013; 22: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


