Abstract
Importance
The use of the rapid antigen‐detection test (RADT) has become the standard of care in the early diagnosis of group A beta‐hemolytic Streptococcus (GAS) pharyngitis. Concern has been expressed over increased false positives when the child had been treated recently for GAS pharyngitis, resulting in over use of antibiotics.
Objective
To determine if the false positive rate for RADT is increased in children recently treated for GAS pharyngitis.
Methods
We conducted a prospective study to evaluate 300 children from a private practice with acute pharyngitis who were treated for GAS pharyngitis within the preceding 28 days (study group) compared to 306 children of comparable age who had not been previously treated (control group). RADT and throat culture were performed on all children presenting with signs and symptoms of acute pharyngitis. The false positive and false negative rates were determined and compared in both groups.
Results
The false positive rate of 11.5% (23/200) in the study group was significantly higher than the false positive rate of 0 in the control group. False positives were more likely to occur in younger children.
Interpretation
These data would indicate that while RADT is reliable in most children, it can lead to over treatment in children who have been recently treated for GAS. In children treated in the preceding 28 days for GAS pharyngitis, the presence of infection should be determined with a throat culture only. Treatment based on a positive RADT should be reserved for children not recently treated for GAS pharyngitis.
Keywords: GAS pharyngitis, Group A beta‐hemolytic Streptococcus rapid antigen‐detection test
INTRODUCTION
Group A beta‐hemolytic Streptococcus (GAS) pharyngitis is one of the most common infections seen in pediatric practice. Failure to diagnose GAS infection and treat it in a timely manner may result in rheumatic fever and rheumatic heart disease. Over the last several decades use of the rapid antigen‐detection test (RADT) has become the standard of care in the early diagnosis of GAS pharyngitis, allowing early treatment to prevent rheumatic fever, decrease transmission of the infection, shorten the course of the disease and prevent complications such as peritonsillar or retropharyngeal abscesses.1 RADT can be performed using various methods. Currently, most commercial tests are immunoassays with a sensitivity of 70%–95% (average 85%) and a specificity of 95%–99%.1, 2, 3, 4, 5, 6 Since RADT detects about 71%–83% of the GAS5, it is a routine of most practices to follow a negative RADT with a GAS culture read at 24 and 48 hours.7 Because of the high specificity of RADT, positives are not typically followed up with a culture on a sheep blood agar plate, first described by Breese and Disney in 1954.8
We have observed that children who have been treated for recent GAS pharyngitis might have a false positive RADT that is rarely seen in children not recently treated. The false positive RADT might be caused by the presence of non‐degraded antigenic proteins that persist even in the absence of viable GAS in the pharynx.5 This seems plausible since the RADT is based on the presence of antigen rather than viable bacteria or antibody. This over diagnosis of GAS pharyngitis would lead to overuse of antibiotics and might result in increased parental anxiety, increased school absenteeism and unnecessary tonsillectomies.
The purpose of this study was to determine if children treated for GAS pharyngitis in the preceding 28 days have a significantly higher false positive rate than children who had not been treated in the preceding 28 days.
METHODS
Clinical study and specimens
This prospective study was conducted in a private pediatric practice in Northern Virginia over a two year period spreading over all seasons. The study was approved by the Institutional review boards (IRB), Georgetown University Medical Center, Washington, DC, USA. The subjects were children and young adults aged 2 to 20 years from middle and upper middle class families living in the Washington, DC suburban area, presenting with signs and symptoms suggestive of acute pharyngitis including sore throat, fever, headache, abdominal pain and cervical lymphadenitis, determined by reviewing the records or taking a history from the parents or older patients. The study group consisted of 300 children who had been treated with an antibiotic for ten days for GAS pharyngitis in the previous 28 days. The control group consisted of 306 children who presented with symptoms suggesting GAS pharyngitis but who had not been treated in the previous 28 days. All subjects had throat cultures regardless of the RADT result.
Antigen tests, culture methods and analysis
Throat swabs were tested by the Osom Strep A test (Sekisui Diagnostics, San Diego, California, USA) for the presence of Strep A antigen (manufacturer's reported sensitivity 96% and specificity 98%), and were also cultured for GAS using 5% sheep blood agar (HealthLink, Jacksonville, Florida, USA) incubated in ambient air at 35 degrees Centigrade for 48 hours. The Osom Strep A test was also used to verify beta hemolytic colonies that grew on the blood agar plate.9 All tests were performed by the same laboratory technicians who were asked to perform a rapid strep test and culture on all subjects without them knowing whether the patient belongs to the study or control group, and checked by the laboratory director. Parental signed consent was obtained and the patients paid no extra charges. Differences in rates were tested using Chi‐square (χ2) analysis with significance set at P < 0.05.
RESULTS
Of three hundred subjects who have been treated with antibiotics for GAS pharyngitis in the past 28 days (study group), 116 (38.7%) were RADT positive. Of these 116 patients, 23 (19.8%) had negative cultures. Seventy percent had been treated with amoxicillin and 30% with a cephalosporin or another antibiotic primarily due to penicillin allergy.
The relative distribution of these according to GAS screen status is shown in Tables 1 and 2. The sensitivity of the GAS screen in these patients was 93.0% while the specificity was 88.5%. Secondary to the rather low specificity, the predictive value of a positive GAS screen was only 80.2% while the high specificity yielded a predictive value of a negative screen at 96.2%. The false negative rate was 7.0% while the false positive rate was relatively high at 11.5%.
Table 1.
Laboratory culture and rapid strep test in patients recently treated for streptococcal pharyngitis
| Group | Number of patients | Culture positive | Culture negative |
|---|---|---|---|
| Rapid strep + | 116 | 93 | 23 |
| Rapid strep ‐ | 184 | 7 | 177 |
| Total | 300 | 100 | 200 |
Table 2.
Sensitivity and specificity of laboratory culture and rapid strep test in patients recently treated for streptococcal pharyngitis
| Variables | Percentage (%) | 95% Confidence Interval (%) |
|---|---|---|
| Sensitivity | 93.0 | 88–98 |
| Specificity | 88.5 | 84–93 |
| Positive Predictive Value | 80.2 | 73–87 |
| Negative Predictive Value | 96.2 | 93–99 |
| False‐positive rate | 11.5 | 8–17 |
| False‐negative rate | 7.0 | 2–12 |
Three hundred and six subjects who had not been treated in the previous 28 days served as controls (control group). Of these children 14.4% were RADT positive. The relative distribution of these according to GAS screen status is shown in Tables 3 and 4. The sensitivity of the rapid strep test in these patients was 80% significantly lower than the study group (χ2 = 5.84, P < 0.05) but the specificity was significantly higher at 100% (χ2 = 27.3, P < 0.01). The higher specificity of the control group also resulted in a significantly higher predictive value for a positive test, 100% for the control group vs. 80.2% for the study group (χ2 = 7.92, P < 0.01). There was no significant difference in the predictive value for a negative test.
Table 3.
Laboratory culture and rapid strep test in patients not recently treated for streptococcal pharyngitis
| Group | Number of patients | Culture positive | Culture negative |
|---|---|---|---|
| Rapid strep + | 44 | 44 | 0 |
| Rapid strep ‐ | 262 | 11 | 251 |
| Total | 306 | 55 | 251 |
Table 4.
Sensitivity and specificity of laboratory culture and rapid strep test in patients not recently treated for streptococcal pharyngitis
| Variables | Percentage (%) | 95% Confidence Interval (%) |
|---|---|---|
| Sensitivity | 80 | 69–91 |
| Specificity | 100 | 99–100 |
| Positive Predictive Value | 100 | 99–100 |
| Negative Predictive Value | 96 | 93–98 |
| False‐positive rate | 0 | 0–0.12 |
| False‐negative rate | 20 | 9–30 |
There is a suggestion that the time from the original infection to new symptoms affected the false positive rate (Figure 1). While the trend was not significant (P = 0.08), there was a significant negative linear relationship between days since infection and the false positive rate (χ2 for non‐linearity = 0.066, P = 0.80). Children under age five were significantly more likely to have false positives than older children (χ2 = 4.7, P < 0.05). There were no false positives in children over 14, but the number of children in this group was small (Table 5). This could not be explained by differences in time to treatment since the time was similar among the age groups (average 14.8–17 days).
Figure 1.
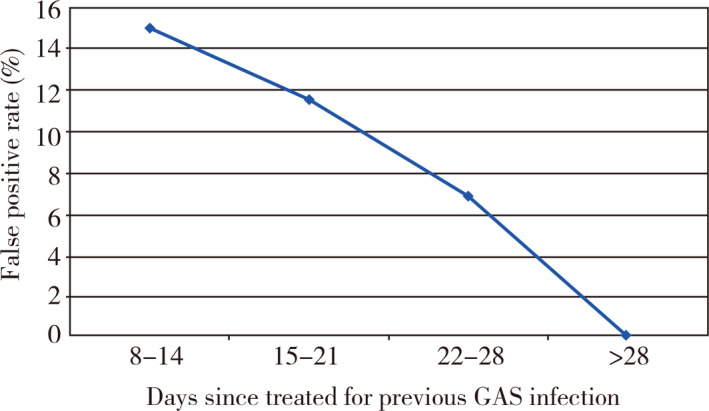
False positive rate by day since completion of the course of treatment
Table 5.
False positive rate by age in study group with previous GAS infection
| Number of patients/Age (year) | True negatives | False positives | False positive rate (%) |
|---|---|---|---|
| 55/2–<5 | 44 | 11 | 20.0 |
| 121/5–<10 | 111 | 10 | 8.2 |
| 16/10–<14 | 14 | 2 | 12.5 |
| 8/≥14 | 8 | 0 | 0 |
DISCUSSION
This study confirms the empiric observation that children recently treated for GAS pharyngitis have a higher rate of false positive rapid strep tests than children not previously treated. While sensitivity was higher in the study group, this did not affect the predictive value of a negative test. Both specificity and positive predictive values were significantly poorer in the previously treated group leading to a relatively high false positive rate and an over diagnosis 11.5% of the time. Since the RADT tests for antigen and not living organisms, this finding is certainly biologically plausible and is supported by the suggestion of a decline in the false positive rate as the time since the last infection increases with no false positives after three weeks. It is also supported by the significantly higher sensitivity in the treated group. If in fact the high false positive rate were due to residual antigen, then a higher sensitivity would be expected since residual antigen would add to the antigen secondary to the new infection.
Sheeler et al5 found that RADT is both sensitive and specific in the setting of recent GAS pharyngitis and they suggested that its use might allow earlier treatment of this subgroup of patients. The previous study was done in an urgent care center of a family practice. While their logistic regression showed no impact of age, they did not describe the population, so it is impossible to determine the age range or number of children they treated in their cohort. Our study was done in a private practice using standard protocols so it should be able to be generalized to other general pediatric practices. The decision to test for GAS was entirely at the discretion of the practicing pediatrician and standard therapy was used. The same laboratory technicians performed all tests using standard methods for this practice. The age distribution was what one would typically see in a general pediatric office.
We found that children less than 5 years old had a significantly higher false positive rate than older children, which might explain the different findings. Although the reason for the difference between their study and ours is not entirely clear, it is possible that this could be explained by differences in immunologic response to GAS infections among children and adults, possibly leading to larger or more persistent antigen loads in children. In conclusion, the high false positive rate of 11.5% in children who were treated within the previous 28 days suggests that while a rapid strep test is reliable in most patients, it could lead to over treatment in children who were treated for GAS within 28 days. While there is a suggestion that this should be applied only to children under 14, more data are needed on older children to confirm this observation. In this era of increasing antibiotic resistance we need to find ways to reduce use of antibiotics. These data would indicate that we should consider using culture alone for children treated for GAS pharyngitis in the previous 28 days and reserve rapid strep testing for children not recently treated for GAS pharyngitis.
CONFLICT OF INTEREST
The authors have no sources of support or conflicts of interest
ACKNOWLEDGMENTS
The authors would like to thank premedical student Grant Frazier for helping with the lab work.
Barakat AJ, Evans C, Gill M, Nelson D. Rapid strep testing in children with recently treated streptococcal pharyngitis. Pediatr Invest. 2019;3:27‐30. 10.1002/ped4.12109
REFERENCES
- 1. Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clin Microbiol Rev. 2004;17:571‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wegner DL, Witte DL, Schrantz RD. Insensitivity of rapid antigen detection methods and single blood agar plate culture for diagnosing streptococcal pharyngitis. JAMA. 1992;267:695‐697. [PubMed] [Google Scholar]
- 3. Fries SM. Diagnosis of group A streptococcal pharyngitis in a private clinic: comparative evaluation of an optical immunoassay method and culture. J Pediatr. 1995;126:933‐936. [DOI] [PubMed] [Google Scholar]
- 4. Schlager TA, Hayden GA, Woods WA, Dudley SM, Hendley JO. Optical immunoassay for rapid detection of group A beta‐hemolytic streptococci. Should culture be replaced? Arch Pediatr Adolesc Med. 1996;150:245‐248. [DOI] [PubMed] [Google Scholar]
- 5. Sheeler RD, Houston MS, Radke S, Dale JC, Adamson SC. Accuracy of rapid strep testing in patients who have had recent streptococcal pharyngitis. J Am Board Fam Pract. 2002;15:261‐265. [PubMed] [Google Scholar]
- 6. Armengol CE, Schlager TA, Hendley JO. Sensitivity of a rapid antigen detection test for group A streptococci in a private pediatric office setting: answering the Red Book's request for validation. Pediatrics. 2004;113:924‐926. [DOI] [PubMed] [Google Scholar]
- 7. Leung AK, Newman R, Kumar A, Davies HD. Rapid antigen detection testing in diagnosing group A β‐hemolytic streptococcal pharyngitis. Expert Rev Mol Diagn. 2006;6:761‐766. [DOI] [PubMed] [Google Scholar]
- 8. Breese BB, Disney FA. The accuracy of diagnosis of beta streptococcal infections on clinical grounds. J Pediatr. 1954;44:670‐673. [DOI] [PubMed] [Google Scholar]
- 9. Rogo T, Schwartz RH, Ascher DP. Comparison of the Inverness Medical Acceava Strep A test with the Genzyme OSOM and Quidel QuickVue Strep A tests. Clin Pediatr (Phila). 2010;49:1050‐1052. [DOI] [PubMed] [Google Scholar]


