Abstract
Importance
The incidence of obstructive sleep apnea syndrome (OSAS) in children has increased year by year recently. Blood pressure research of OSAS children can help understand the occurrence of OSAS related complications. Early detection and intervention of blood pressure changes in children with OSAS can reduce the incidence of cardiovascular disease in later adulthood.
Objective
To investigate the differences in blood pressure among different groups of snoring children and different sleep stages.
Methods
Habitually snoring children (snoring frequency of ≥ 3 nights per week) aged 3 to 11 years were recruited from Beijing Children's Hospital from 1 January 2017 to 30 June 2018. All children underwent polysomnography, and their blood pressure was monitored and calculated by the pulse transit time. The children were divided into those with primary snoring (PS), mild OSAS, and moderate to severe OSAS according to their obstructive apnea–hypopnea index (OAHI).
Results
In total, 140 children were included. Ninety‐seven had PS, 24 had mild OSAS, and 19 had moderate to severe OSAS. There were no differences in age, sex, or body mass index z‐score among the groups. Statistically significant differences were found in the OAHI, oxygen desaturation index 3%, respiratory arousal index, and lowest oxygen saturation among the three groups. Children with moderate to severe OSAS had higher systolic and diastolic blood pressure than those with mild OSAS and PS (P < 0.001). In all children, systolic and diastolic blood pressure was higher in the rapid eye movement (REM) sleep stage than in the non‐REM sleep stage (P < 0.05).
Interpretation
Children with moderate to severe OSAS had higher blood pressure than those with PS and mild OSAS in all sleep stages. Blood pressure in the REM sleep stage was higher than that in other sleep stages in all groups of children.
Keywords: Blood pressure, Children, Obstructive sleep apnea syndrome (OSAS)
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a common type of sleep‐disordered breathing caused by repeated upper airway collapse during sleep, which results in frequent arousals, hypoxemia, hypercapnia, and sleep structure changes. OSAS can lead to a variety of complications including insulin resistance, dyslipidemia, metabolic syndrome, as well as cardiovascular injuries (e.g., hypertension, endothelial dysfunction, and pulmonary hypertension).1
Hypertension is a common complication of OSAS, which adversely affects overall health. Many studies have demonstrated that children with OSAS have higher blood pressure than those without OSAS.2, 3 Further research has shown that preschool children with OSAS undergo changes in blood pressure during the rapid eye movement (REM) stage of sleep4; in addition, blood pressure may be normal during the day but elevated at night, and a study of children aged 5–14 years showed that blood pressure regulation may be abnormal in children with OSAS.5 After treatment, the systolic blood pressure remarkably decreases in children with OSAS.6 High blood pressure in childhood has been shown to increase the risks of hypertension, coronary heart disease, and other diseases in adulthood.7 Early detection of blood pressure changes in children with OSAS and appropriate intervention can effectively reduce the incidence of cardiovascular disease in adulthood and lower the disease burden.
Traditional blood pressure monitoring is performed using a cuff, stethoscope, and mercury sphygmomanometer. However, this technique is uncomfortable for many patients and cannot effectively reveal the actual blood pressure in each patient during nighttime sleep. With the introduction of polysomnography (PSG) in recent years, the pulse transit time (PTT) has become the most widely used blood pressure monitoring parameter.8 The PTT is defined as the time taken for the arterial pulse pressure wave to travel from the left ventricle to a predetermined peripheral site. The mean arterial blood pressure and the distance between the two sites affect the PTT. In patients with high blood pressure, the stiffness of the blood vessel wall increases, the pulse passage velocity increases, and the PTT decreases.9 Notably, measurement of the PTT does not require a cuff. As a noninvasive and continuous technique, the PTT can allow collection of multiple blood pressure values and better reflect blood pressure throughout the night, thus enabling the early detection of blood pressure changes in pediatric patients. This technique is quite comfortable and has only a small impact on sleep.10 Previous studies have confirmed that blood pressure values monitored by measurement of the PTT are highly consistent with those obtained by traditional ambulatory blood pressure monitoring.11
The present study was designed to investigate the differences in blood pressure among children with different severities of OSAS and among different sleep stages.
METHODS
Patients
Children aged 3 to 11 years who presented to our sleep center for evaluation of habitual snoring and underwent overnight PSG from 1 January 2017 to 30 June 2018 were consecutively enrolled in this study. The parents of all children and children who were older than 8 years provided written informed consent. This study was approved by the ethics committee of Beijing Children's Hospital.
The exclusion criteria were obesity [body mass index (BMI) z‐score of ≥ 1.65]; current use of drugs that affect sleep, respiration, blood pressure, and cognitive function; accompanying diseases that can cause high blood pressure, such as heart and kidney disease; craniofacial malformations; inherited metabolic diseases; neuromuscular diseases; and a family history of hypertension.
Physical examination
Staff at the sleep center measured the heights and weights of all children using standard methods (accurate to 0.1 kg or 0.1 cm). BMI was calculated as body weight (kg) / height2 (m2). The BMI z‐score was calculated based on age, weight, and height (http://zscore.research.chop.edu/index.php).
PSG
All children underwent overnight PS G and PTT monitoring at our center using the SOMNOscreen Plus PSG+ V5 system (SOMNOmedics GmbH, Randersacker, Bavaria, Germany). All children fell asleep under natural conditions. Ingestion of coffee, tea, cola, and sedative/hypnotic drugs was forbidden 4 hours before sleep. The sleep time was more than 7 hours. PSG monitoring included four‐lead electroencephalography (C3/A2, C4/ A1, O1/A2, and O2/A3), bilateral electrooculography, mandibular electromyography, electrocardiography, monitoring of chest and abdominal breathing, oxygenation saturation, use of an airflow thermistor and nasal pressure cannula, use of a snore sensor, and monitoring of body position. Respiratory events during sleep were evaluated in accordance with the American Academy of Sleep Medicine scoring manual.12 The sleep data were evaluated by experienced monitoring staff in the sleep center.
The obstructive apnea–hypopnea index (OAHI) refers to the number of episodes of obstructive apnea, mixed apnea, and hypopnea per hour of sleep. The oxygen desaturation index 3% refers to the number of times that an average 3% reduction in arterial oxygen saturation occurs per hour of sleep.12 According to clinical practice guidelines for the diagnosis and management of childhood OSAS, primary snoring (PS) is defined as an OAHI of < 1, childhood OSAS is defined as an OAHI of ≥ 1, mild OSAS is defined as an OAHI of ≥ 1 to < 5, and moderate to severe OSAS is defined as an OAHI of ≥ 5.13
PTT method
Blood pressure was measured by the PTT method. Specifically, electrocardiography monitoring was applied to monitor changes in cardiac electricity; a fingertip pulse oximeter was used to monitor pulse wave changes at the fingertip. The PTT is defined as the time required for the arterial pulse pressure wave to travel from the left ventricle (peak of the electrocardiography R wave) to a predetermined peripheral site (peak point of the pulse wave). The distance from the left ventricle to the fingertip is positively correlated with the body height. Therefore, pulse‐related blood pressure can be calculated based on the PTT and body height; blood pressure data can be obtained for each beat.9 In the present study, blood pressure data were recorded every 30 seconds to monitor changes with respect to individual sleep stages.
Statistical analysis
Normally distributed data are expressed as mean ± standard deviation and were compared among the three study groups using analysis of variance. Non‐normally distributed data are expressed as median with interquartile range and were compared using the Wilcoxon rank‐sum test. All statistical analyses were performed using the SPSS 23.0 statistical software package (IBM Corp., Armonk, NY, USA), and a P value of < 0.05 was considered statistically significant. Figures and tables were created using JMP 11.0 software (SAS Institute, Cary, NC, USA).
RESULTS
A total of 208 children met the initial inclusion criteria; of these, 61 children were excluded because they had a BMI z‐score of ≥ 1.65, while seven were excluded because their data were incomplete. In total, 140 children were included in the final analysis.
Comparison of general conditions, PSg findings, and blood pressure among the three study groups
Based on the PSG findings, 97 children had PS, 24 children had mild OSAS, and 19 children had moderate to severe OSAS. The mean ages were 6.8 ± 2.1 years, 6.5 ± 1.9 years, and 7.0 ± 2.1 years in the PS, mild OSAS, and moderate to severe OSAS groups, respectively. The number of boys was 59 (60.8%), 18 (75.0%), and 15 (78.9%) in the PS, mild OSAS, and moderate to severe OSAS groups, respectively. There were no significant differences in age, sex, BMI z‐score, height, total sleep time, or sleep efficiency among the three groups (P > 0.05) (Table 1). Human sleep can be divided into four stages: N1, N2, N3, and REM. The N1 of total sleep time significantly differed between the PS and moderate to severe OSAS groups (6.2% vs. 9.8%, P < 0.05); the N2 and N3 of total sleep time significantly also differed between the PS and moderate to severe OSAS groups, as well as between the mild OSAS and moderate to severe OSAS groups (all P < 0.05). The REM of total sleep time significantly differed between the mild OSAS and moderate to severe OSAS groups (P < 0.05). In addition, the OAHI, oxygen desaturation index 3%, respiratory arousal index, and minimum oxygen saturation significantly differed among the three groups (all P < 0.05). The overall systolic and diastolic blood pressures also significantly differed among the three groups (both P < 0.05) (Table 1).
Table 1.
Demographic and clinical characteristics of the three study groups
| Items | PS group (n = 97) | Mild OSAS group (n = 24) | Moderate and severe OSAS group (n = 19) | P |
|---|---|---|---|---|
| Age (years) | 6.8 ± 2.1 | 6.5 ± 1.9 | 7.0 ± 2.1 | > 0.05 |
| Male, n (%) | 59 (60.8) | 18 (75.0) | 15 (78.9) | > 0.05 |
| BMI z‐value | −0.42 ± 1.22 | −0.32 ± 1.51 | −0.18 ± 0.88 | > 0.05 |
| Height (cm) | 123.3 ± 13.8 | 122.0 ± 11.7 | 126.3 ± 13.2 | > 0.05 |
| OAHI | 0.0 (0.0–0.3) | 1.8 (1.1–2.9) | 10.1 (6.0–20.5) | < 0.05† , ‡ , § |
| ODI3% | 1.1 (0.5–2.1) | 4.5 (2.4–9.6) | 26.5 (13.2–69.7) | < 0.05† , ‡ , § |
| Respiratory arousal index | 0.7 (0.3–1.6) | 3.5 (2.1–6.0) | 10.3 (5.9–18.2) | < 0.05† , ‡ , § |
| Minimum oxygen saturation (%) | 91 (91–94) | 90 (88–92) | 80 (73–86) | < 0.05† , ‡ , § |
| TST (h) | 8.06 (7.28–8.52) | 8.27 (7.70–8.81) | 8.40 (7.45–9.02) | > 0.05 |
| Sleep efficiency (%) | 86.4 (77.4–91.8) | 89.5 (75.5–93.1) | 87.1 (79.5–93.7) | > 0.05 |
| N1 of TST (%) | 6.2 (4.4–9.1) | 6.3 (4.9–10.3) | 9.8 (6.6–11.3) | < 0.05‡ |
| N2 of TST (%) | 49.2 (45.0–53.1) | 49.3 (41.7–52.5) | 52.3 (50.1–59.2) | < 0.05 ‡ , § |
| N3 of TST (%) | 24.8 (22.0–28.6) | 26.2 (22.3–28.2) | 21.7 (15.1–24.2) | < 0.05‡ , § |
| REM of TST (%) | 18.3 (15.5–20.8) | 18.9 (16.6–21.1) | 16.3 (12.2–19.6) | < 0.05§ |
| Systolic blood pressure (mmHg) | 105.0 ± 11.3 | 103.0 ± 12.7 | 107.1 ± 13.9 | <0.05† , ‡ , § |
| Diastolic blood pressure (mmHg) | 70.1 ± 10.0 | 67.6 ± 9.7 | 71.7 ± 11.1 | <0.05† , ‡ , § |
Data are presented as mean ± SD or n (%) or median (interquartile range). † P < 0.05 between PS and mild OSAS groups; ‡ P < 0.05 between PS and moderate to severe OSAS groups; § P < 0.05 between mild OSAS and moderate to severe OSAS groups. PS, primary snoring; OSAS, obstructive sleep apnea syndrome; OAHI, obstructive apnea–hypopnea index; ODI3%, oxygen desaturation index 3%; BMI, body mass index; TST, total sleep time; REM, rapid eye movement.
Comparison of systolic and diastolic blood pressure among different sleep stages
Compared with the mild OSAS and PS groups, the moderate to severe OSAS group had significantly higher systolic and diastolic blood pressures in sleep stages N1, N2, N3, and REM (all P < 0.001) (Figures 1 and 2). Compared with the PS group, the mild OSAS group had significantly lower systolic and diastolic blood pressures in all sleep stages (P < 0.001) (Figures 1 and 2).
Figure 1.
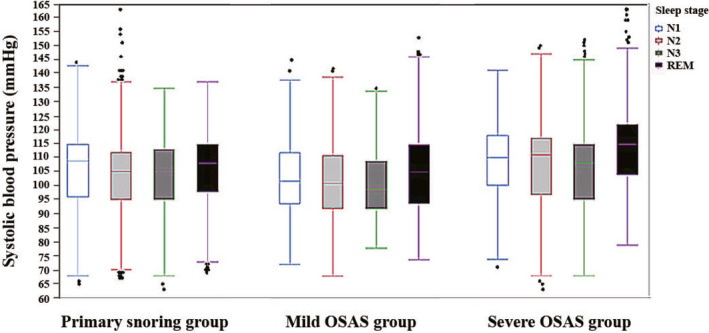
Comparison of systolic blood pressures in all sleep stages among the primary snoring, mild OSAS, and moderate to severe OSAS groups. OSAS, obstructive sleep apnea syndrome. REM, rapid eye movement
Figure 2.
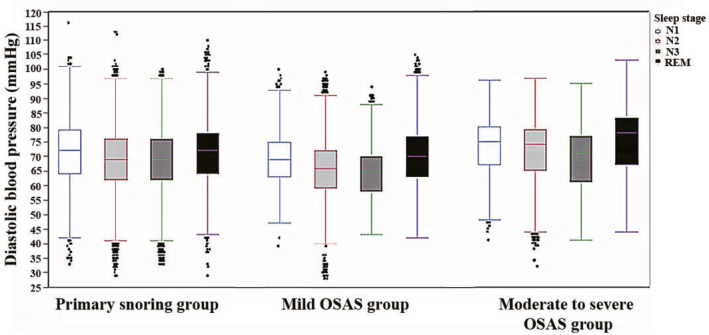
Comparison of diastolic blood pressures in all sleep stages among the primary snoring, mild OSAS, and moderate to severe OSAS groups. OSAS, obstructive sleep apnea syndrome. REM, rapid eye movement
DISCUSSION
OSAS is a common childhood sleep disorder that seriously impacts the health of affected children. Previous surveys have shown that the prevalence of OSAS ranges from 1.2% to 5.7%.14 Epidemiological studies have demonstrated that OSAS is an important factor in the development of hypertension.15 In adults, OSAS is an important independent risk factor for hypertension, and the severity of OSAS is positively correlated with blood pressure.16 Notably, blood pressure has been shown to improve after treatment of OSAS. Many studies have suggested that children with OSAS have higher blood pressure than those without OSAS; moreover, children with moderate to severe OSAS have a higher risk of elevated nighttime blood pressure.2, 3 Xu et al5 conducted PSG and ambulatory blood pressure monitoring in children with snoring and found no significant difference in blood pressure between children with and without OSAS during the daytime; however, during the night, both systolic and diastolic blood pressures were significantly higher in children with OSAS than in children without OSAS. A study in which the PTT was used to monitor blood pressure in Australian preschool children showed that children with moderate to severe OSAS had elevated blood pressure during the REM stage of sleep.14 These previous studies indicate that blood pressure in children with OSAS increases at night and during the REM stage of sleep.
In the present study, we used the PTT method to monitor the children's nighttime blood pressure. Unlike traditional monitoring techniques, the PTT method does not affect sleep and allows collection of a larger number of blood pressure values; in addition, the blood pressure values collected by the PTT method correspond with sleep stages in a real‐time manner and therefore more accurately reflect the actual nighttime blood pressure.10 Furthermore, obese children were excluded from our study, thus ruling out the impact of obesity on blood pressure. Our study showed that children with moderate to severe OSAS had significantly higher blood pressure in each sleep stage, relative to children with PS or mild OSAS; the children's blood pressure was higher in the REM stage than in the other sleep stages in all observation groups (all P < 0.05). If blood pressure is measured only at an outpatient visit during the daytime, changes in blood pressure that occur in children with OSAS may not be appropriately recorded. We presume that, although children with OSAS have an impaired cardiovascular system, its clinical manifestations are subtle. Persistent abnormal nighttime blood pressure will eventually lead to the development of true hypertension in the future, even in adulthood.15 Delayed intervention in children with OSAS may result in a missed window of opportunity for early treatment of cardiovascular complications.
The mechanism of cardiovascular system impairment in children with OSAS may include several aspects. First, repeated airway obstruction in children with OSAS may result in hypoxemia, hypercapnia, and multiple arousals during sleep, leading to increased nighttime sympathetic excitability and inhibited parasympathetic excitability. Over an extended period, autonomic nerve regulatory function will be impaired, heart rate variability will increase, and both systolic and diastolic blood pressures will increase.4, 14 In addition, increased sympathetic excitability will improve the levels of catecholamines in the body, cause arterioles to shrink, increase peripheral vascular resistance, and increase heart rate and blood pressure.16 Second, oxygen free radicals can increase blood vessel tension and cause vasospasms, thereby raising blood pressure. Oxygen free radicals can also cause a systemic inflammatory response, which increases blood pressure.17 In patients with OSAS, monocytes expressing CD15 and CD11c adhesion molecules were reportedly increased, which could result in higher levels of oxygen free radicals. Furthermore, long‐term hypoxemia can cause oxidative stress and produce oxygen free radicals.18 Third, the inflammatory response and insulin resistance can cause increased hypertension in patients with OSAS. Moreover, in a study of patients with equivalent OSAS severity, insulin levels and inflammatory indicators were both higher in those with hypertension than in those without hypertension.19
Human sleep can be divided into four stages: N1, N2, N3, and REM. Among them, stages N1, N2, and N3 are collectively known as non‐REM sleep. In the present study, blood pressures in different sleep stages were analyzed. Notably, blood pressure was significantly higher in the REM stage than in non‐REM stages in all observation groups (all P < 0.05). Previous studies have shown that respiratory events typically occur in the REM stage because of the sensory dysfunction, reduced muscle tone, and increased arousal threshold in this period,20 which may explain why blood pressure was more pronounced in the REM stage than in other sleep stages in this study. In contrast, the N3 stage is a deep sleep period during which parasympathetic nerves predominate and both blood pressure and heart rate tend to be slow and stable.4 Accordingly, among all sleep stages, blood pressure was lowest in the N3 stage in this study.
Our study had some limitations. First, obese children were excluded from analysis to rule out the impact of obesity on blood pressure. Importantly, many children with OSAS are obese, and the exclusion of these patients from our study resulted in a much larger number of children in the PS group than in the mild OSAS and moderate to severe OSAS groups; thus, there was an imbalance in the sample size. Second, our study lacked a control group of non‐ snoring children. Third, our study lacked follow‐up data; thus, it remains unclear whether blood pressure in these children was improved after treatment.
In conclusion, children with moderate to severe OSAS had higher blood pressure than children with PS in all stages of sleep; the blood pressure in both groups was highest in the REM stage. Thus, we presume that children with OSAS have an impaired cardiovascular system. Early changes in blood pressure should be monitored in children with OSAS, and appropriate interventions should be implemented to lower the incidence of future cardiovascular disease.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Geng X, Wu Y, Ge W, Feng G, Zheng L, Xu Z, et al. Ambulatory blood pressure monitoring in children with obstructive sleep apnea syndrome. Pediatr Invest. 2019;3:217‐222. 10.1002/ped4.12163
Funding source
The Pediatric Medical Coordinated Development Center of Beijing Hospitals Authority (XTYB201807); Capital Health Research and Development of Special Funding (2018‐1‐2091); National Key Research and Development Plan (2017YFC0112502)
Contributor Information
Zhifei Xu, Email: zhifeixu@aliyun.com.
Xin Ni, Email: nixin@bch.com.cn.
REFERENCES
- 1. Blechner M, Williamson AA. Consequences of obstructive sleep apnea in children. Curr Probl Pediatr Adolesc Health Care. 2016;46:19‐26. [DOI] [PubMed] [Google Scholar]
- 2. Horne RS, Yang JS, Walter LM, Richardson HL, O'Driscoll DM, Foster AM, et al. Elevated blood pressure during sleep and wake in children with sleep‐disordered breathing. Pediatrics. 2011;128:85‐92. [DOI] [PubMed] [Google Scholar]
- 3. Li AM, Au CT, Sung RY, Ho C, Ng PC, Fok TF, et al. Ambulatory blood pressure in children with obstructive sleep apnea: a community based study. Thorax. 2008;63:803‐809. [DOI] [PubMed] [Google Scholar]
- 4. Nisbet LC, Yiallourou SR, Biggs SN, Nixon GM, Davey MJ, Trinder JA, et al. Preschool children with obstructive sleep apnea: The beginnings of elevated blood pressure? Sleep. 2013;36:1219‐1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu Z, Li B, Shen K. Ambulatory blood pressure monitoring in Chinese children with obstructive sleep apnea/hypopnea syndrome. Pediatr Pulmonol. 2013;48:274‐279. [DOI] [PubMed] [Google Scholar]
- 6. Ng DK, Wong JC, Chan CH, Leung LC, Leung SY, et al. Ambulatory blood pressure before and after adenotonsillectomy in children with obstructive sleep apnea. Sleep Med. 2010;11:721‐725. [DOI] [PubMed] [Google Scholar]
- 7. Lou XH, Wang MM, Xi B. Effect of childhood high blood pressure on the risk of hypertension in adulthood. Chin J Child Health Care. 2019;27:584‐587. (in Chinese) [Google Scholar]
- 8. Smith LA, Dawes PJ, Galland BC. The use of pulse transmit time in pediatric sleep studies: A systematic review. Sleep Med Rev. 2018;37:4‐13. [DOI] [PubMed] [Google Scholar]
- 9. Mukkamala R, Hahn JO, Inan OT, Mestha LK, Kim CS, Toreyin H, et al. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans Biomed Eng. 2015;62:879‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foo JY, Wilson SJ. Clinical applications of pulse transit time in pediatric critical care. J Med Eng Technol. 2009;33:79‐86. [DOI] [PubMed] [Google Scholar]
- 11. Gómez García MT, Troncoso Acevedo MF, Rodriguez Guzmán M, Alegre de Montaner R, Fernández Fernández B, del Río Camacho G, et al. Can pulse transit time be useful for detecting hypertension in patients in a sleep unit? Arch Bronconeumol. 2014;50:278‐84. [DOI] [PubMed] [Google Scholar]
- 12. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714‐755. [DOI] [PubMed] [Google Scholar]
- 14. Walter LM, Yiallourou SR, Vlahandonis A, Sands SA, Johnson CA, Nixon GM, et al. Impaired blood pressure control in children with obstructive sleep apnea. Sleep Med. 2013;14:858‐866. [DOI] [PubMed] [Google Scholar]
- 15. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342:1378‐1384. [DOI] [PubMed] [Google Scholar]
- 16. Mohsenin V. Obstructive sleep apnea and hypertension: a critical review. Curr Hypertens Rep. 2014;16:482. [DOI] [PubMed] [Google Scholar]
- 17. Brandes RP. Endothelial dysfunction and hypertension. Hypertension. 2014;64:924‐928. [DOI] [PubMed] [Google Scholar]
- 18. Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934‐939. [DOI] [PubMed] [Google Scholar]
- 19. Qian X, Yin T, Li T, Kang C. High levels of inflammation and insulin resistance in obstructive sleep apnea patients with hypertension. Inflammation. 2012;35:1507‐1511. [DOI] [PubMed] [Google Scholar]
- 20. Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension: An update. Hypertension. 2014;63:203‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katz ES, Marcus CL, White DP. Influence of airway pressure on genioglossusactivity during sleep in normal children. Am J Respir Crit Care Med. 2006;173:902‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]


