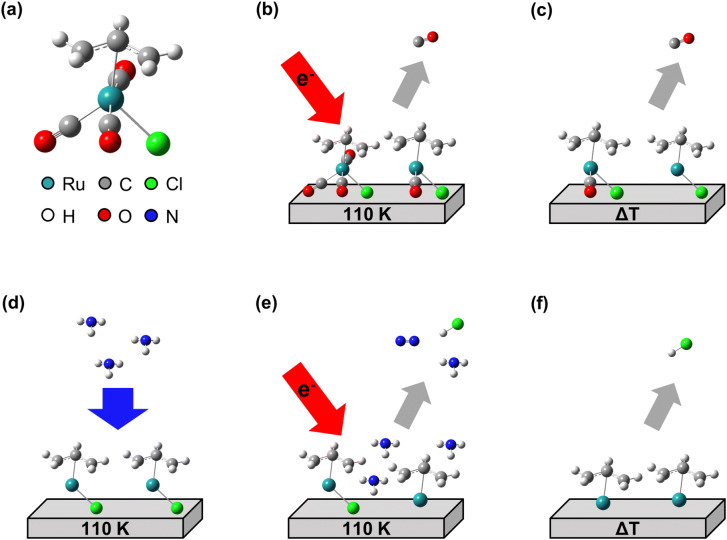
Figure 1.
Overview of experiments to unravel the chemistry that underlies the electron-induced formation of deposits from the Ru precursor (η3-C3H5)Ru(CO)3Cl and the NH3-assisted purification process aiming at removal of Cl from the deposits. (a) Structure of the precursor and colour scheme of the relevant elements. Deposits were produced by (b) electron-stimulated desorption (ESD) from thin condensed layers of (η3-C3H5)Ru(CO)3Cl on Ta held at 110 K and (c) subsequent thermal desorption spectrometry (TDS) during which the sample was annealed to 450 K at a rate of 1 K/s. The deposit was used in the subsequent purification experiments consisting of several cycles in each of which (d) NH3 was condensed onto the deposit at 110 K, followed by (e) an ESD and (f) a TDS experiment. Desorption of volatile species was monitored in all experiments by mass spectrometry (MS).