Abstract
Recognition of viral dsRNA by Toll-like receptor 3 (TLR3) leads to the induction of downstream antiviral effectors and the innate antiviral immune response. Here, we identified the zinc-finger FYVE domain-containing protein ZFYVE1, a guanylate-binding protein (GBP), as a positive regulator of TLR3-mediated signaling. Overexpression of ZFYVE1 promoted the transcription of downstream antiviral genes upon stimulation with the synthetic TLR3 ligand poly(I:C). Conversely, ZFYVE1 deficiency had the opposite effect. Zfyve1−/− mice were less susceptible than wild-type mice to inflammatory death induced by poly(I:C) but not LPS. ZFYVE1 was associated with TLR3, and the FYVE domain of ZFYVE1 and the ectodomain of TLR3 were shown to be responsible for their interaction. ZFYVE1 was bound to poly(I:C) and increased the binding affinity of TLR3 to poly(I:C). These findings suggest that ZFYVE1 plays an important role in the TLR3-mediated innate immune and inflammatory responses by promoting the ligand binding of TLR3.
Keywords: TLR3, ZFYVE1, ligand binding, immune response
Subject terms: Toll-like receptors, Cell signalling
Introduction
Toll-like receptors (TLRs) are a family of type I integral membrane-associated receptors that recognize structurally conserved microbial components called pathogen-associated molecular patterns, leading to the induction of the innate immune and inflammatory responses.1 TLRs are distinct in their cellular localization, ligand recognition, and utilization of signaling adapter molecules, which contributes to functional specificities.2
TLR3 recognizes viral dsRNA and is expressed in a variety of cell types, such as dendritic cells (DCs), macrophages, and fibroblasts.3 TLR3 is localized in intracellular compartments, such as the ER and early endosomes. It has also been reported that TLR3 is localized on the plasma membrane in human fibroblasts.4 TLR3 contains an N-terminal leucine-rich repeat (LRR) domain (the ectodomain), a transmembrane domain, and a C-terminal cytoplasmic Toll/IL-1 receptor (TIR) domain. The LRR ectodomain of TLR3 is responsible for recognizing dsRNA at least 40–50 base pairs in length.5 H539 and N541 have been shown to be two key dsRNA-binding residues in TLR3.6 Multimerization of TLR3 and an acidic pH are required for TLR3 ligand binding.7,8 The TIR domain of TLR3 recruits downstream TIR domain-containing adapter inducing IFN-β (TRIF) after the binding of TLR3 to ligand.9
TLR3 is synthesized in the ER and then translocated to endosomes, where it is cleaved by cathepsins B and H. The processed fragments of TLR3 form a stable complex that is capable of binding to its ligand and initiating downstream signaling. In addition, the stimulation of TLR3 by its ligand has been shown to cause its upregulation.10–12 Recently, Mex3B was reported to be a coreceptor of TLR3 that promotes proteolytic processing and TLR3 ligand binding.3,13 Upon binding to its ligand, TLR3 is phosphorylated by protein tyrosine kinases, including EGFR and SRC, which are essential for recruitment of the critical downstream adapter protein TRIF by TLR3.9,10,12,14 TRIF in turn recruits the TRAF3-TBK1/IKKε and TRAF6-IKKα/β/γ complexes to activate the transcriptional factors IRF3/7 and NF-κB, respectively, followed by the translocation of these transcriptional factors into the nucleus and ultimately the induction of type I interferon (IFN), proinflammatory cytokine, and other downstream effector antiviral genes.15,16
In this study, we identified ZFYVE1, a guanylate-binding protein that contains a zinc-finger FYVE domain, as a new positive regulator of TLR3-mediated signaling. We found that ZFYVE1 is associated with TLR3 and promotes the binding of TLR3 to its ligand. ZFYVE1 deficiency inhibited the TLR3- mediated innate immune and inflammatory responses, but not innate immune and inflammatory responses mediated by TLR4. Our findings suggest that ZFYVE1 is a cofactor for ligand recognition by TLR3, which plays an important role in the innate antiviral response.
Results
Identification of ZFYVE1 as a positive regulator of TLR3-mediated signaling
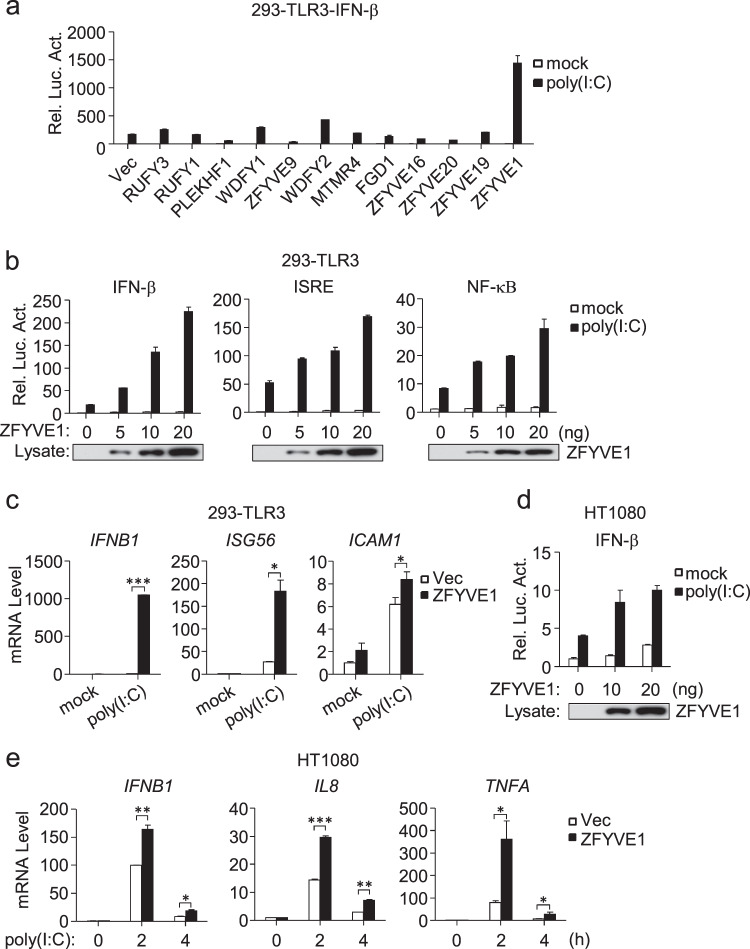
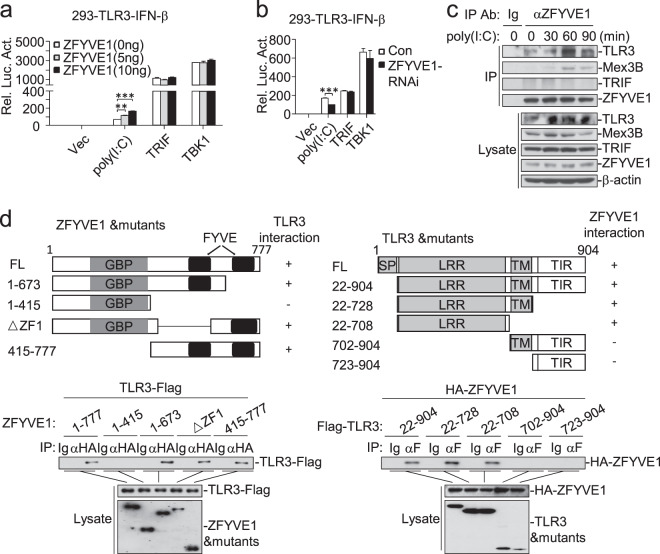
Previously, we reported that the zinc-finger FYVE domain-containing protein WDFY1 potentiates TLR3/4-mediated signaling by facilitating recruitment of the adapter TRIF to these receptors.17 We further screened the effects of 12 other zinc-finger FYVE domain-containing proteins on TLR3-mediated signaling and identified ZFYVE1 as the candidate protein with the strongest stimulatory effect on poly(I:C)-triggered activation of the IFN-β promoter in 293-TLR3 cells (Fig. 1a). Overexpression of ZFYVE1 potentiated poly(I:C)-induced activation of the IFN-β promoter, ISRE, and NF-κB in a dose-dependent manner in 293-TLR3 cells (Fig. 1b). Quantitative PCR (qPCR) experiments indicated that the overexpression of ZFYVE1 potentiated poly(I:C)-induced transcription of the IFNB1, ISG56, and ICAM1 genes (Fig. 1c). In similar assays, the overexpression of ZFYVE1 also potentiated poly(I:C)-induced activation of the IFN-β promoter and the transcription of downstream genes in HT1080 cells (Fig. 1d, e) that respond to extracellular poly(I:C) stimulation.9 These results suggest that overexpression of ZFYVE1 potentiates TLR3-mediated signaling.
Fig. 1.
Identification of ZFYVE1 as a positive regulator of poly(I:C)-triggered signaling. a Effects of zinc-finger FYVE domain-containing proteins on TLR3-mediated signaling. 293-TLR3 cells (1 × 105) were seeded on 24-well plates and transfected with IFN-β reporter (0.1 μg) and pRL-TK Renilla luciferase reporter plasmid (0.01 μg) along with the indicated plasmids (0.1 μg each) for 18 h and then were or were not treated with poly(I:C) (20 μg/mL) for 6 h. Luciferase assays were performed with a dual-specific luciferase assay kit. Firefly luciferase activities were normalized against Renilla luciferase activities. b Effects of ZFYVE1 on poly(I:C)-induced activation of the IFN-β promoter, ISRE, and NF-κB. 293-TLR3 cells (1 × 105) were transfected with the indicated reporter and increasing amounts of the HA-ZFYVE1 plasmid for 18 h and were or were not treated with poly(I:C) (20 μg/mL) for 6 h before luciferase assays. The lower blots show the expression levels of transfected ZFYVE1 as detected by anti-Flag antibody. c Effects of ZFYVE1 on the poly(I:C)-induced transcription of downstream genes. 293-TLR3 cells (1 × 105) were transfected with either empty plasmid or the HA-ZFYVE1 plasmid (0.2 μg) for 18 h and then were or were not treated with poly(I:C) (30 μg/mL) for 3 h before qPCR analysis. d Effects of ZFYVE1 on poly(I:C)-induced activation of the IFN-β promoter in HT1080 cells. Luciferase assays were performed as described in b except for the use of HT1080 cells. e Effects of ZFYVE1 on the poly(I:C)-induced transcription of downstream genes in HT1080 cells. Control and HT1080 cells stably expressing ZFYVE1-Flag were or were not treated with poly(I:C) (50 μg/mL) for the indicated times before qPCR analysis. Data are represented as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test)
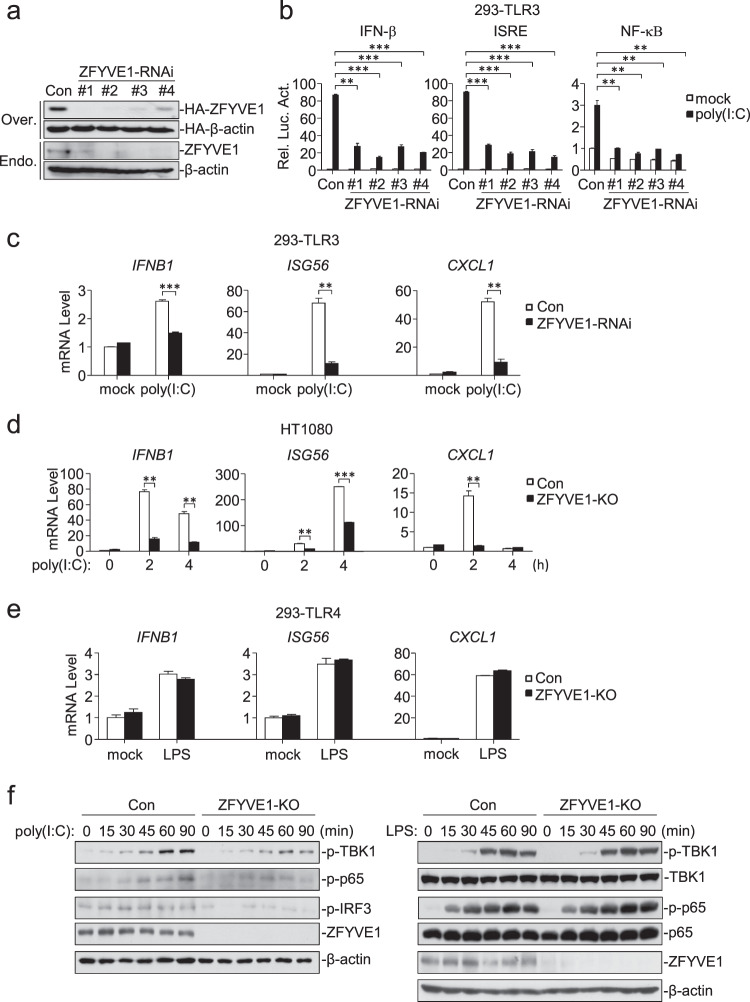
To determine the roles of endogenous ZFYVE1 in TLR3-mediated signaling, we constructed four ZFYVE1-RNAi plasmids in which the expression of transfected and endogenous ZFYVE1 was inhibited (Fig. 2a). Knockdown of ZFYVE1 inhibited poly(I:C)-induced activation of the IFN-β promoter, ISRE, and NF-κB in 293-TLR3 cells (Fig. 2b) (we selected the ZFYVE1-RNAi-#1 construct for additional experiments.). qPCR experiments indicated that knockdown of ZFYVE1 inhibited the poly(I:C)-induced transcription of the IFNB1, ISG56, and CXCL1 genes (Fig. 2c). We also generated ZFYVE1-deficient HT1080 and 293-TLR4 cells by the CRISPER/Cas9 method. qPCR experiments showed that ZFYVE1 deficiency inhibited the poly(I:C)-induced, but not LPS-induced, transcription of the IFNB1, ISG56, and CXCL1 genes (Fig. 2d, e). Consistently, the poly(I:C)-induced, but not LPS-induced, phosphorylation of TBK1, IRF3, and p65, which is a hallmark of IRF3 and NF-κB activation, was impaired in ZFYVE1-deficient cells compared with that in control cells (Fig. 2f). These results suggest that ZFYVE1 is an important regulator of TLR3-mediated signaling.
Fig. 2.
ZFYVE1 is important for TLR3-mediated signaling. a Effects of ZFYVE1-RNAi on the expression of ZFYVE1. As shown in the upper two panels, HEK293 cells (1 × 105) were transfected with HA-ZFYVE1 (0.1 μg), HA-β-actin (0.01 μg), and the indicated ZFYVE1-RNAi plasmids (0.5 μg) for 24 h before immunoblotting analysis. As shown in the lower two panels, HEK293 cells (1 × 105) were transfected with the indicated ZFYVE1-RNAi plasmids (0.5 μg) for 12 h. The cells were then selected with puromycin (1 μg/mL) for 24 h before immunoblotting analysis. b Effects of ZFYVE1-RNAi on poly(I:C)-induced activation of the IFN-β promoter, ISRE, and NF-κB. 293-TLR3 cells (1 × 105) were transfected with the indicated reporter and ZFYVE1-RNAi plasmids (0.5 μg) for 36 h and then were or were not treated with poly(I:C) (20 μg/mL) for 6 h before luciferase assays. c Effects of ZFYVE1-RNAi on the poly(I:C)-induced transcription of downstream genes. 293-TLR3 cells (1 × 105) were transfected with control or ZFYVE1-RNAi plasmid (1 μg) for 12 h. The cells were selected with puromycin (1 μg/mL) for 24 h and then were or were not treated with poly(I:C) (50 μg/mL) for 3 h before qPCR analysis. d Effects of ZFYVE1 deficiency on the poly(I:C)-induced transcription of downstream genes. ZFYVE1-deficient HT1080 cells were generated by the CRISPR-Cas9 method. ZFYVE1-KO and control HT1080 cells (1 × 105) were or were not treated with poly(I:C) (50 μg/mL) for the indicated times before qPCR analysis. e Effects of ZFYVE1 deficiency on the LPS-induced transcription of downstream genes. ZFYVE1-deficient 293-TLR4 cells were generated by the CRISPR-Cas9 method. ZFYVE1-KO and control 293-TLR4 cells (1 × 105) were or were not treated with LPS (100 ng/mL) for 2 h before qPCR analysis. f Effects of ZFYVE1 deficiency on the poly(I:C)- and LPS-induced phosphorylation of TBK1, IRF3, and p65. ZFYVE1-KO and control cells (1 × 105) were or were not treated with poly(I:C) (50 μg/mL) or LPS (100 ng/mL) for the indicated times before immunoblotting analysis. Data are represented as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test)
Zfyve1 deficiency attenuates TLR3-mediated signaling in primary mouse cells
To further confirm the roles of ZFYVE1 in vivo, we generated Zfyve1-deficient mice by the CRISPR/Cas9 method (Supplementary Fig. S1a).18,19 The successful generation of Zfyve1-deficient mice was verified by genotyping, qPCR, and immunoblotting analysis (Supplementary Fig. S1b–d). The homozygous Zfyve1−/− mice were born at the Mendelian ratio and exhibited normal growth and development (Supplementary Fig. S1e), which suggests that Zfyve1 is dispensable for mouse development and survival. In addition, the compositions of various immune cells in the thymus, spleen, and lymph nodes in Zfyve1−/− mice and their wild-type littermates were comparable (Supplementary Fig. S2a, b), suggesting that Zfyve1 is not required for the development and maturation of various types of immune cells.
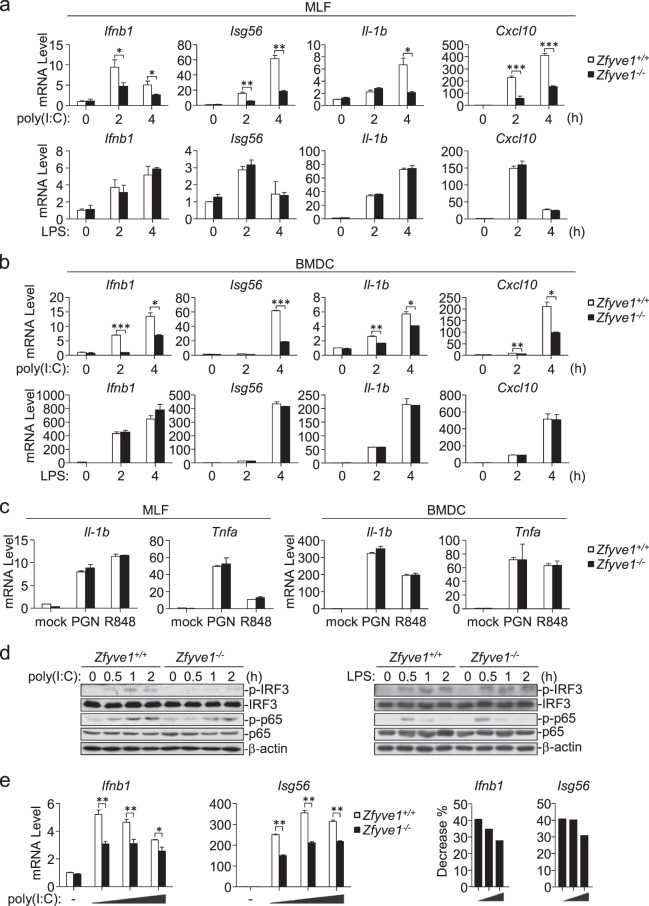
To determine whether Zfyve1 is involved in the TLR3-mediated innate immune response, we examined the expression of downstream genes induced by the indicated stimuli in Zfyve1+/+ and Zfyve1−/− mouse lung fibroblasts (MLFs) and bone marrow-derived DCs (BMDCs). qPCR experiments showed that poly(I:C)-induced, but not LPS-induced, transcription of the Ifnb1, Isg56, Cxcl10 and Il1b genes was severely impaired in Zfyve1−/− MLFs and BMDCs in comparison with their wild-type counterparts (Fig. 3a, b). In addition, Zfyve1 deficiency had no obvious effects on the peptidoglycan (PGN, a synthetic TLR2 ligand)- or R848 (a synthetic TLR7/8 ligand)-induced transcription of the Il1b and Tnfa genes in MLFs and BMDCs (Fig. 3c). Consistently, the poly(I:C)-induced, but not LPS-induced, phosphorylation of IRF3 and p65 were impaired in Zfyve1−/− compared with that in wild-type BMDCs (Fig. 3d). These data suggest that Zfyve1 specifically potentiates TLR3-mediated signaling in primary mouse cells.
Fig. 3.
Zfyve1 deficiency attenuates TLR3-mediated signaling in primary mouse cells. a Effects of Zfyve1 deficiency on the poly(I:C)- and LPS-induced transcription of downstream genes. Zfyve1+/+ and Zfyve1−/− MLFs (2 × 105) were or were not treated with poly(I:C) (50 μg/mL) or LPS (100 ng/mL) for the indicated times before qPCR analysis. b Effects of Zfyve1 deficiency on the poly(I:C)- and LPS-induced transcription of downstream genes. Zfyve1+/+ and Zfyve1−/− BMDCs (2 × 105) were or were not treated with poly(I:C) (20 μg/mL) or LPS (100 ng/mL) for the indicated times before qPCR analysis. c Effects of Zfyve1 deficiency on the PGN- or R848-induced transcription of downstream genes. Zfyve1+/+ and Zfyve1−/− MLFs or BMDCs (2 × 105) were or were not treated with PGN (20 mg/mL) or R848 (10 mM) for 3 h before qPCR analysis. d Effects of Zfyve1 deficiency on the poly(I:C)-induced phosphorylation of IRF3 and p65. Zfyve1+/+ and Zfyve1−/− BMDCs (2 × 105) were or were not treated with poly(I:C) (50 μg/mL) or LPS (100 ng/mL) for the indicated times before immunoblotting analysis. e Effects of Zfyve1 deficiency on the transcription of downstream genes induced by different doses of poly(I:C). Zfyve1+/+ and Zfyve1−/− MLFs (2 × 105) were or were not treated with increasing amounts of poly(I:C) for 3 h before qPCR analysis. The percentage reduction in the transcription of the Ifnb1 and Isg56 genes in Zfyve1−/− MLFs compared with that in Zfyve1+/+ MLFs is shown in the right panels. Data are represented as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test)
We further investigated transcription of the Ifnb1 and Isg56 genes induced by increasing amounts of poly(I:C) in Zfyve1+/+ and Zfyve1−/− MLFs. The poly(I:C)-induced transcription of the Ifnb1 and Isg56 genes was inhibited in Zfyve1−/− MLFs compared with that in wild-type MLFs, which was more dramatic when lower doses of poly(I:C) were added (Fig. 3e). These results suggest that ZFYVE1 plays a more dramatic role in TLR3-mediated signaling when stimulated with a low concentration of poly(I:C).
Zfyve1 deficiency impairs the TLR3-mediated innate immune and inflammatory responses in mice
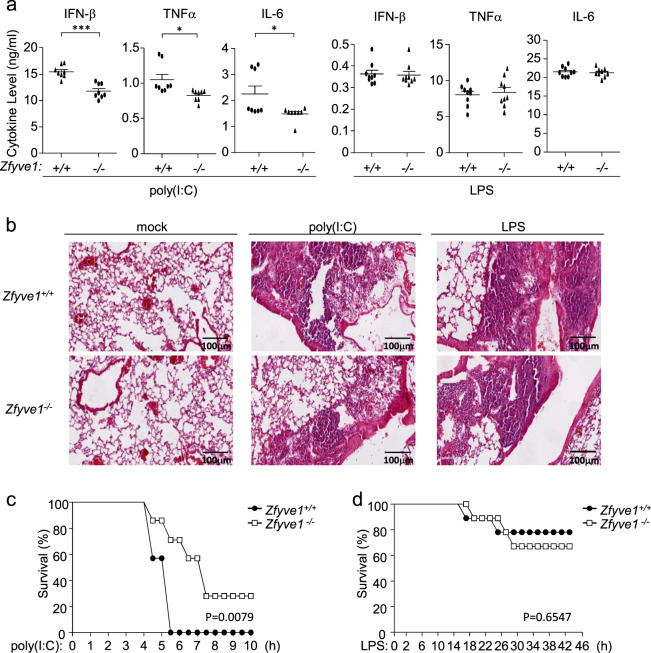
To further explore the roles of ZFYVE1 in the regulation of the TLR3-mediated innate immune and inflammatory responses in vivo, age- and sex-matched Zfyve1+/+ and Zfyve1−/− mice were intraperitoneally (i.p.) injected with poly(I:C) plus D-galactosamine or LPS. As shown in Fig. 4a, Zfyve1−/− mice injected with poly(I:C) had lower levels of serum IFN-β, TNFα, and IL-6 than wild-type mice, whereas LPS-induced serum cytokine levels in Zfyve1+/+ and Zfyve1−/− mice were comparable. Hematoxylin and eosin staining indicated that poly(I:C)-induced, but not LPS-induced, inflammatory damage in the lungs was relieved in Zfyve1−/− mice (Fig. 4b). Zfyve1−/− mice were less susceptible to inflammatory death induced by poly(I:C), but not LPS, than wild-type mice (Fig. 4c, d). These results suggest that ZFYVE1 potentiates TLR3-mediated innate immune and inflammatory responses in mice.
Fig. 4.
Zfyve1 deficiency impairs TLR3-mediated innate immune and inflammatory responses in mice. a Effects of Zfyve1 deficiency on serum levels of IFN-β, TNFα, and IL-6 induced by poly(I:C) and LPS. Sex- and age-matched Zfyve1+/+ and Zfyve1−/− mice (n = 8) were injected intraperitoneally with poly(I:C) (1 μg/g) plus D-galactosamine (0.5 mg/g) or LPS (10 μg/g) for 4 h before measurement of the indicated serum cytokines by ELISA. Each symbol represents an individual mouse. b Effects of Zfyve1 deficiency on poly(I:C)- and LPS-induced inflammation. Sex- and age-matched Zfyve1+/+ and Zfyve1−/− mice were injected intraperitoneally with poly(I:C) (1 μg/g) plus D-galactosamine (0.5 mg/g) or LPS (10 μg/g) for 4 h, and lung sections were used for histological analysis (H&E staining). Scale bars, 200 μm. c, d Effects of Zfyve1 deficiency on poly(I:C)- and LPS-induced death in mice. Sex- and age-matched Zfyve1+/+ and Zfyve1−/− mice (n = 8) were injected intraperitoneally with poly(I:C) (1 μg/g) plus D-galactosamine (0.5 mg/g) or LPS (10 μg/g), and the survival of mice was monitored every 0.5 h over the following 2 days. Data are represented as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test)
ZFYVE1 is associated with TLR3
We next investigated the molecular mechanisms responsible for the roles of ZFYVE1 in TLR3-mediated signaling. Reporter assays showed that overexpression of ZFYVE1 potentiated poly(I:C)-induced, but not TRIF- and TBK1-induced, activation of the IFN-β promoter in a dose-dependent manner (Fig. 5a), whereas knockdown of ZFYVE1 inhibited signaling triggered by poly(I:C) stimulation but not the overexpression of TRIF and TBK1 (Fig. 5b). Endogenous coimmunoprecipitation experiments indicated that ZFYVE1 is associated with TLR3, but not TRIF or Mex3B, in unstimulated cells. Poly(I:C) stimulation increased the association of ZFYVE1 with TLR3 and induced the association of ZFYVE1 with Mex3B, which peaked at 60 min post stimulation and then decreased (Fig. 5c). Domain mapping experiments indicated that the LRR-containing ectodomain of TLR3 and the C-terminal tandem FYVE domains of ZFYVE1 were responsible for their association (Fig. 5d).
Fig. 5.
ZFYVE1 is associated with TLR3. a Effects of ZFYVE1 on activation of the IFN-β promoter triggered by poly(I:C) and TRIF or TBK1. 293-TLR3 cells were transfected with the IFN-β reporter and increasing amounts of HA-ZFYVE1 plasmid for 18 h and then were or were not treated with poly(I:C) (20 μg/mL) for 6 h before luciferase assays. In addition, 293-TLR3 cells were transfected with the IFN-β reporter, TRIF or TBK1, and increasing amounts of HA-ZFYVE1 plasmid for 24 h before luciferase assays. b Effects of ZFYVE1-RNAi on activation of the IFN-β promoter triggered by poly(I:C), TRIF, or TBK1. 293-TLR3 cells (1 × 105) were transfected with the IFN-β reporter and either a control or ZFYVE1-RNAi plasmid. Transfected cells were selected with puromycin (1 μg/mL) for 24 h, followed by either treatment with poly(I:C) (20 μg/mL) or retransfection with the TRIF or TBK1 plasmids. Luciferase assays were performed 6 h after treatment or 18 h after retransfection. c Endogenous ZFYVE1 is associated with TLR3. HT1080 cells (4 × 107) were or were not treated with poly(I:C) (100 μg/mL) for the indicated times before coimmunoprecipitation and immunoblotting analysis. d Domain mapping between ZFYVE1 and TLR3. HEK293 cells (2 × 106) were transfected with the indicated plasmids for 20 h before coimmunoprecipitation and immunoblotting analysis. Data are represented as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 (Student’s t test)
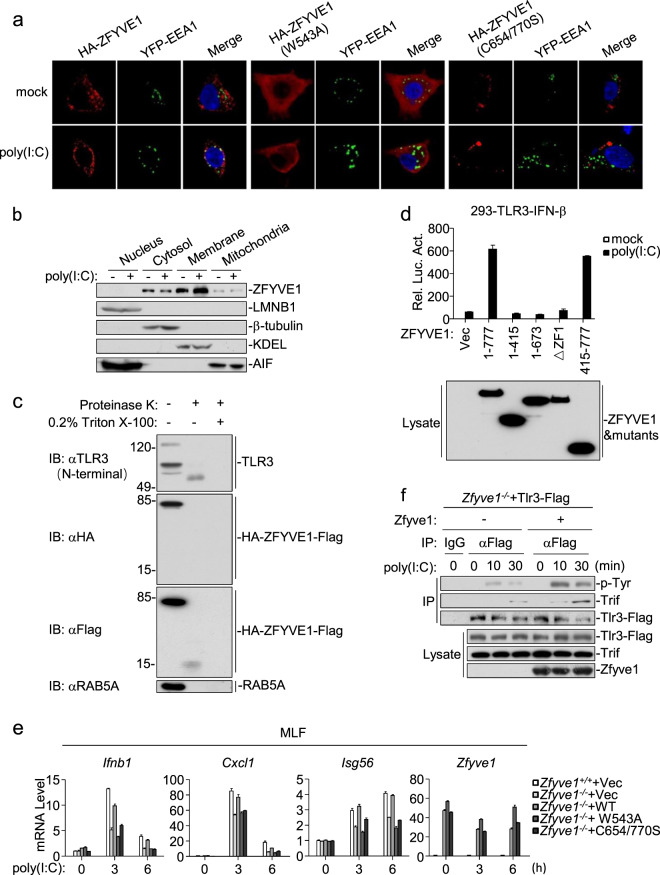
ZFYVE1 is located in the ER, mitochondria, and Golgi apparatus but not in endosomes.20,21 Confocal microscopy confirmed these observations. However, poly(I:C) stimulation caused partial colocalization of ZFYVE1 with endosomes (Fig. 6a and Supplementary Fig. S3a). We further analyzed the subcellular localization of endogenous ZFYVE1 by cellular fractionation assays. ZFYVE1 was detected in the mitochondrial, membrane, and cytosolic fractions but not in the nuclear fraction in unstimulated cells, and poly(I:C) stimulation promoted the translocation of ZFYVE1 from the cytosolic to membrane fraction (Fig. 6b). It has been reported that the FYVE domain can insert into phosphatidylinositol 3-phosphate (PI3P)-enriched membranes and that the extent of this membrane penetration is increased in acidic media.22 As shown by confocal microscopy, two ZFYVE1 mutants, ZFYVE1 (C654/770S), in which two critical cysteine residues in the FYVE zinc fingers are mutated, and ZFYVE1 (W543A), a mutant that lacks the ability to penetrate the membrane, were not localized to endosomes after poly(I:C) stimulation (Fig. 6a and Supplementary Fig. S3a). To determine whether ZFYVE1 can be sorted or inserted into the endosomal lumen, we generated 293-TLR3 cells stably expressing N-terminal HA- and C-terminal Flag-tagged ZFYVE1 (HA-ZFYVE1-Flag). We purified the endosomes and performed proteinase K protection assays. The C-terminal FYVE domain containing a fragment of HA-ZFYVE1-Flag, which was detected as an ~15-kDa band, and the N-terminal ectodomain of TLR3 were protected in proteinase K degradation assays (Fig. 6c). These results suggest that both the C-terminal FYVE domains of ZFYVE1 and the ectodomain of TLR3 are located on the luminal side of endosomes. Consistently, overexpression of ZFYVE (415-777) was sufficient to potentiate poly(I:C)-triggered activation of the IFN-β promoter in 293-TLR3 cells (Fig. 6d).
Fig. 6.
Poly(I:C) stimulation causes the partial colocalization of ZFYVE1 with endosomes. a Subcellular localization of ZFYVE1 and its mutants. HEK293 cells (1 × 105) were transfected with HA-tagged ZFYVE1, ZFYVE1 (W543A) or ZFYVE1 (C654/770S) plus YFP-EEA1 (early endosomal marker). Twenty hours later, the cells were or were not treated with poly(I:C) (50 μg/mL) for 20 min. The cells were then fixed with 4% paraformaldehyde and stained with anti-ZFYVE1 antibody before confocal microscopy. b Subcellular localizations of endogenous ZFYVE1. 293-TLR3 cells (2 × 107) were or were not treated with poly(I:C) (100 μg/mL) for 30 min. Cellular fractions were analyzed by immunoblotting with the indicated antibodies. c The C-terminal FYVE domains of ZFYVE1 are located at the endosomal lumen. 293-TLR3 cells stably expressing HA-ZFYVE1-Flag (3 × 108) were treated with poly(I:C) (100 μg/mL) for 30 min. Endosome/lysosome fractions were isolated and either left untreated, treated with proteinase K (2 μg/mL), or treated with proteinase K plus 0.2% Triton X-100 for 15 min at 55 °C. The samples were then analyzed by immunoblotting with the indicated antibodies. d Effects of ZFYVE1 and its mutants on poly(I:C)-induced activation of the IFN-β promoter. 293-TLR3 cells (1 × 105) were transfected with the IFN-β reporter and the indicated plasmids for 24 h and then were or were not treated with poly(I:C) (20 μg/mL) for 6 h before luciferase assays. Expression of transfected ZFYVE1 and its mutants was detected by immunoblotting analysis. e Effects of reconstitution with ZFYVE1, ZFYVE1 (W543A) or ZFYVE1 (C654/770S) on the poly(I:C)-induced transcription of downstream antiviral genes. Zfyve1−/− MLFs were reconstituted with murine ZFYVE1, ZFYVE1 (W543A), or ZFYVE1 (C654/770) by lentiviral-mediated gene transfer. The reconstituted MLFs were or were not treated with poly(I:C) for the indicated times before qPCR analysis. f Zfyve1 deficiency inhibits the poly(I:C)-induced tyrosine phosphorylation of TLR3. Zfyve1-deficient MLFs stably expressing Tlr3-Flag were reconstituted with vector or Zfyve1, and the cells (4 × 107) were or were not treated with poly(I:C) (100 μg/mL) for the indicated times before coimmunoprecipitation and immunoblotting analysis
ZFYVE1 is a PtdIns3P (PI3P)-binding protein, and both FYVE domains are required for its binding to PI3P.23 Although ZFYVE (1-673) bound to poly(I:C) and TLR3 with similar affinity, it failed to potentiate poly(I:C)-triggered activation of IFN-β (Fig. 6d). One explanation for this result is that ZFYVE (1-673) lacks the second FYVE domain and therefore has no PI3P-binding activity. Consistently, three PI3P-binding defective mutants, ZFYVE1 (C654S), ZFYVE1 (C770S), and ZFYVE1 (C654/770S), could not potentiate poly(I:C)-triggered activation of the IFN-β promoter in reporter assays (Supplementary Fig. S3b). In addition, reconstitution of wild-type, but not W543A or C654/770S ZFYVE1 into ZFYVE1-deficient MLFs restored the poly(I:C)-triggered induction of downstream antiviral genes (Fig. 6e). Reconstitution of ZFYVE1 also rescued the poly(I:C)-triggered tyrosine phosphorylation of TLR3 in Zfyve1-deficient MLFs (Fig. 6f).
ZFYVE1 facilitates the binding of TLR3 to poly(I:C)
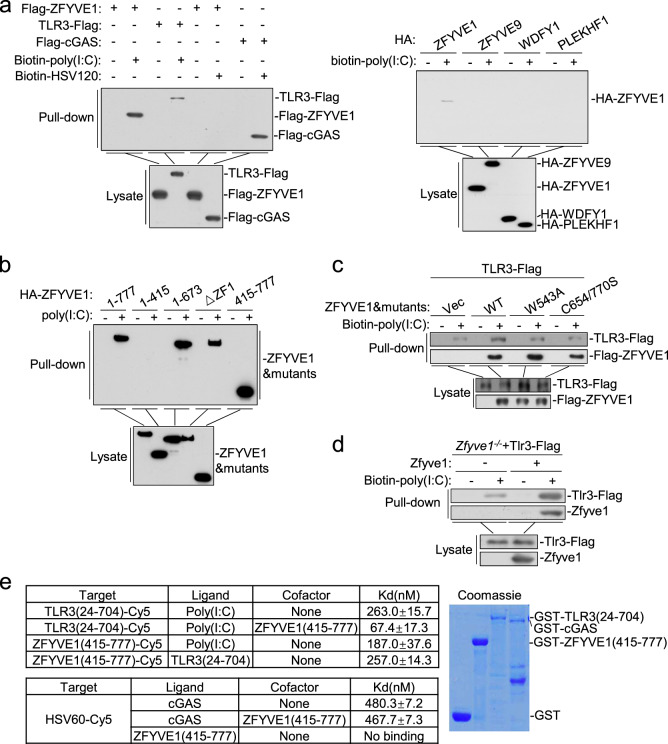
Since ZFYVE1 is a zinc-finger domain-containing protein predicted to bind nucleic acids,23 we determined whether ZFYVE1 binds to poly(I:C). Pulldown experiments showed that ectopically expressed ZFYVE1, but not the other tested FYVE domain-containing proteins ZFYVE9, WDFY1, and PLEKHF1, could bind to poly(I:C) but not the synthetic DNA HSV120 (a ligand for cGAS) (Fig. 7a). Deletion analysis indicated that the two FYVE domains could independently bind to poly(I:C) (Fig. 7b). Pulldown analysis indicated that ZFYVE1 enhanced the binding of TLR3 to poly(I:C). In these experiments, ZFYVE1 (C654/770S) and ZFYVE1 (W543A) had no marked effects on the binding of TLR3 to poly(I:C) (Fig. 7c). Conversely, Zfyve1 deficiency impaired the binding of TLR3 to poly(I:C) in MLFs (Fig. 7d). We further produced recombinant ZFYVE1 (415-777) and TLR3 (24-704) and examined their binding affinities to poly(I:C) by microscale thermophoresis technology (MST). ZFYVE1 (415-777) bound to poly(I:C) with a slightly higher affinity than TLR3 did (Kd = 187.0 and 263.0 nM, respectively). Interestingly, the binding affinity of the ZFYVE1 and TLR3 complex to poly(I:C) (Kd = 67.4 nM) was markedly higher than that of each component alone (Fig. 7e). In these experiments, ZFYVE (415-777) did not bind to the synthetic DNA HSV60 and had no marked effect on the binding affinity of cGAS to HSV60 (Fig. 7e). These results suggest that ZFYVE1 promotes the binding of TLR3 to its ligand.
Fig. 7.
ZFYVE1 facilitates the binding of TLR3 to poly(I:C). a ZFYVE1 binds to poly(I:C) but not HSV120. HEK293 cells (2 × 106) were transfected with the indicated plasmids. Eighteen hours later, cell lysates were incubated with biotinylated poly(I:C) or biotinylated HSV120 and streptavidin sepharose beads for in vitro pulldown assays. The bound proteins were analyzed by immunoblotting with anti-Flag or anti-HA antibody. b ZFYVE1 binds to poly(I:C) through its FYVE domains. HEK293 cells (2 × 106) were transfected with the indicated plasmids. Eighteen hours later, cells were collected for in vitro pulldown assays performed as described in a. c Wild-type, but not W543A or C654/770S ZFYVE1 enhances the binding of poly(I:C) to TLR3. HEK293 cells (2 × 106) were transfected with the indicated plasmids. Eighteen hours later, cells were collected for in vitro pulldown assays performed as described in a. d Zfyve1 deficiency inhibits the binding of poly(I:C) to TLR3. Zfyve1-deficient MLFs stably expressing Tlr3-Flag were reconstituted with vector or murine ZFYVE1, and the cell lysates (2 × 108 each) were then incubated with biotinylated poly(I:C) and streptavidin sepharose beads for in vitro pulldown assays. The bound proteins were then analyzed by immunoblotting with anti-Flag antibody. e ZFYVE1 increases the binding affinity of poly(I:C) to TLR3. Binding affinities between the indicated recombinant proteins or between the indicated recombinant proteins and poly(I:C) were measured by MST. The purified recombinant proteins were stained with coomassie blue (right panel)
Discussion
TLR3, an endosomal localized TLR that recognizes viral dsRNA, plays a critical role in the activation of the host immune response to viral infections.24 How TLR3 specifically and efficiently recognizes viral dsRNA remains poorly understood. In this study, we identified ZFYVE1 as a coreceptor of TLR3 important for dsRNA-induced innate immune and inflammatory responses.
Several lines of evidence support the critical role of ZFYVE1 in TLR3-mediated signaling. The overexpression of ZFYVE1 markedly potentiated poly(I:C)-induced activation of IRF3 and NF-κB, whereas ZFYVE1 deficiency had the opposite effect. ZFYVE1 deficiency inhibited the poly(I:C)-induced, but not LPS-induced, expression of innate immune and inflammatory genes in cells and mice. Zfyve1−/− mice were less susceptible than wild-type mice to poly(I:C)-induced, but not LPS-induced, inflammatory damage and death. These results demonstrate the important role of ZFYVE1 in TLR3-mediated innate immune and inflammatory responses.
Our studies suggest that ZFYVE1 modulates TLR3-mediated signaling by its physiological and functional association with TLR3. Coimmunoprecipitation experiments indicated that ZFYVE1 is associated with TLR3 by its FYVE domains, and their association was increased following poly(I:C) stimulation. Proteinase K degradation assays indicated that the C-terminal FYVE domains of ZFYVE1 and the ectodomain of TLR3 were localized to the luminal side of endosomes. In vitro pulldown analysis indicated that ZFYVE1 binds to poly(I:C) through its FYVE domains. Overexpression of ZFYVE1 promoted the binding of TLR3 to poly(I:C), whereas Zfyve1 deficiency had the opposite effect. MST analysis with purified recombinant proteins indicated that ZFYVE1 binds to poly(I:C) with a slightly higher affinity than TLR3 does and markedly increases the binding affinity of TLR3 to poly(I:C). Consistently, overexpression of ZFYVE (415-777) was sufficient to potentiate poly(I:C)-triggered activation of the IFN-β promoter in 293-TLR3 cells, whereas reconstitution of wild-type, but not W543A or C654/770S ZFYVE1 into ZFYVE1-deficient MLFs restored the poly(I:C)-triggered induction of downstream antiviral genes. In addition, ZFYVE1 deficiency had a more dramatic effect on the binding of TLR3 to low concentrations of poly(I:C), which is consistent with the role of ZFYVE1 in enhancing the ligand binding of TLR3. Collectively, our results suggest that ZFYVE1 acts as a coreceptor for the binding of TLR3 to its ligand.
WDFY1, another FYVE domain-containing protein, has been reported to potentiate TLR3/4-mediated signaling by mediating recruitment of the adapter protein TRIF to these receptors. In contrast, we found that ZFYVE1 potentiated TLR3-mediated, but not TLR4-mediated, signaling. ZFYVE1, but not WDFY1, had the ability to bind to poly(I:C) and acted as a coreceptor for TLR3. The FYVE domain of ZFYVE1 is located in endosomal lumen, where it interacts with the ectodomain of TLR3. In contrast, WDFY1 associated with TLR3 and TLR4 in a stimulation-dependent manner and mediated the recruitment of the adapter protein TRIF through its WD domains. These studies suggest that ZFYVE1 and WDFY1 modulate TLR3-mediated signaling through distinct mechanisms.
Mex3B, another poly(I:C)-binding protein, acts as a TLR3 coreceptor by promoting the ligand binding and proteolytic processing of TLR3.3,13 Whether ZFYVE1 and Mex3B collaborate to promote ligand recognition by TLR3 remains to be investigated. Alternatively, these two coreceptors may act by distinct mechanisms during processing, complex formation, stabilization, and ligand binding. Nevertheless, our study reveals new mechanisms by which a TLR3 cofactor recognizes TLR3 binding to its ligand.
Materials and methods
Reagents, antibodies, and cells
The following reagents, antibodies, and cells were purchased from the indicated companies: TRIzol (TaKaRa Bio); SYBR Green (Bio-Rad); a dual-specific luciferase assay kit (Promega); polybrene (Millipore); poly(I:C), PGN, and R848 (Invivogen); LPS and DNase I (Sigma); EZ-link Psoralen-PEG3-Biotin (Thermo), type II collagenase (Worthington), a first strand cDNA synthesis kit (Fermentas), GammaBind G Plus-Sepharose (Amersham Biosciences), a cell mitochondria isolation kit (Beyotime), a nuclear and cytoplasmic extraction kit (Applygen); ELISA kits to detect murine IFN-β, TNFα, and IL-6 (BioLegend); mouse monoclonal antibodies against Flag, β-actin (Sigma), HA (Origene), p-IRF3 (Cell Signaling Technology), p-p65 (Cell Signaling Technology), TLR3 (Cell Signaling Technology), p-Tyr (Cell Signaling Technology), IRF3 (Santa Cruz Biotechnology), AIF (Santa Cruz Biotechnology), KDEL (Santa Cruz Biotechnology), p65 (Abcam), TBK1 (Abcam), p-TBK1 (Abcam), TRIF (Abcam), LMNB1 (Proteintech), β-tubulin (Life Technology), ZFYVE1 and RAB5A (Abclonal); and Alexa Fluor 488- and Alexa Fluor 594-conjugated goat anti-mouse IgG antibodies (Invitrogen). Mouse polyclonal antibodies against Mex3B (Dr Yanyi Wang, Wuhan Institute of Virology, China), 293-TLR3 cells (Dr Katherine Fitzgerald, University of Massachusetts Medical School, USA), and 293-TLR4 cells (Dr Hongliang Li, Wuhan University, China) were obtained from the indicated investigators. HT1080 cells (China Center for Type Culture Collection) were purchased from the indicated organizations.
Constructs
Mammalian expression plasmids for Flag- or HA-tagged ZFYVE1, TLR3, and their mutants were constructed by standard molecular biology techniques. Flag-tagged TRIF, TBK1, cGAS, HA-tagged β-actin, IFN-β promoter, ISRE, NF-κB reporter plasmids, GFP-KDEL (ER marker), YFP-GM130 (Golgi marker), YFP-EEA1 (early endosomal marker), and YFP-TOM20 (mitochondria marker) were previously described.3,25,26 The pSuper.Retro RNAi plasmid was purchased from Oligoengine, Inc.
ZFYVE1 knockout mice and genotyping
Zfyve1 gene knockout mice with a C57BL/6J background were generated utilizing the CRISPR/Cas9 system by the Wuhan University Animal Center. The strategy used to construct the targeting vector is illustrated in Supplementary Fig. S1a. Exon 3 of ZFYVE1 was targeted by specific gRNAs, which resulted in a deletion in exon 3 (nt 484-988) of the mouse Zfyve1 coding sequence.
Genotyping was performed by PCR using the following pairs of primers:
1F: TCTGGTGAGTAGACAGTGGTG, 1R: AGGCATAATCACTCTGCCTGG;
2F: AGCTGATTGCTGAAGTGAAAC, and 2R: AGGCATAATCACTCTGCCTGG.
Amplification of the WT allele with primer pair 1 resulted in a 250-bp fragment, whereas amplification of the disrupted allele with primer pair 2 resulted in a 450-bp fragment.
All animal experiments were performed in accordance with the Wuhan University Animal Care and Use Committee guidelines.
Generation of BMDCs
Bone marrow cells were isolated from the tibiae and femurs of mice. To prepare BMDCs, bone marrow cells (1 × 107) were cultured in RPMI 1640 medium supplemented with 10% FBS and murine GM-CSF (10 ng/mL) for 6–9 days.
Isolation of MLFs
Primary MLFs were isolated from ~4- to 6-week-old mice as described.27,28 The lungs were minced and digested in calcium- and magnesium-free HBSS containing 10-μg/mL type II collagenase and 20-μg/mL DNase I for 3 h at 37 °C with shaking. Cell suspensions were filtered through progressively smaller cell filters (100 and 40 μm) and then centrifuged at 1500 rpm for 4 min. The cells were then plated in culture medium (1:1 [v/v] DMEM/Ham’s F-12 containing 10% FBS, 15-mM HEPES, 2-mM L-glutamine, 50-U/mL penicillin, and 50-μg/mL streptomycin). One hour later, adherent fibroblasts were rinsed with HBSS and cultured in medium.
Cell lines and retroviral gene transfer
The 293 cells plated on 100-mm dishes were transfected with the indicated retroviral plasmids (10 μg) with the pGag-pol (10 μg) and pVSV-G (3 μg) plasmids. After 2 days of transfection, the viruses were harvested and used to infect 293-TLR3 or HT1080 cells in the presence of polybrene (8 μg/mL). The infected cells were selected with puromycin (1 μg/mL) for at least 6 days before experiments.
Transfection and reporter assays
The cells were transfected by standard calcium phosphate precipitation. To normalize for the transfection efficiency, pRL-TK (Renilla luciferase) reporter plasmid (0.02 μg) was added to each transfection. An empty control plasmid was added to ensure that each transfection received the same amount of total plasmid DNA. Eighteen hours after transfection, the cells were or were not treated with the indicated stimulus before luciferase assays performed using a dual-specific luciferase assay kit. Firefly luciferase activities were normalized based on Renilla luciferase activities.
RNAi experiments
Double-stranded oligonucleotides corresponding to the target sequences were cloned into the pSuper.Retro RNAi plasmid. The following human ZFYVE1 mRNA sequences were targeted:
#1: GCGGGACTGATGAAGCTAT, #2: GCATAACGACCTCTTCAAA;
#3: GCGACGTCCTTTAAAGATA, and #4: GCGGACAGAGATTGTGCAT.
Quantitative PCR
Total RNA was isolated from cells using TRIzol reagent.29,30 After reverse transcription with oligo(dT) primer using a RevertAidTM First Strand cDNA Synthesis Kit, the samples were subjected to qPCR analysis to measure the mRNA expression levels of the tested genes. The data shown are the relative abundance of the indicated mRNAs normalized to that of GAPDH or Gapdh. The sequences of qPCR primers were previously reported29,31,32 or are listed below:
human ZFYVE1: GTGCCCAAAACATCTGCTTCCAC, ACTGCCGACTACGATAGACCAC;
murine Zfyve1: GAACGAGGACATACAATCCGCC and ACCGCTGAAGCGGTCACTTAGT.
Confocal microscopy
293-TLR3 cells transfected with the indicated plasmids were or were not treated with poly(I:C) (50 μg/mL) for 30 min, fixed with 4% paraformaldehyde for 10 min, and permeabilized with 0.1% Triton X-100 in PBS for 15 min. The cells were blocked with 1% BSA in PBS and stained with the indicated primary and secondary antibodies for 1 h. The nuclei were stained with DAPI. The cells were observed with a Zeiss confocal microscope with a 60× objective.
In vitro pulldown assays
Poly(I:C) was conjugated to biotin by UV (365-nm wavelength) crosslinking for 1 h. The 293 cells transfected with the indicated plasmids were lysed in lysis buffer (20-mM Tris-HCl, pH 6.0, 150-mM NaCl, 1-mM EDTA, and 1% NP-40). The lysates were incubated with biotinylated poly(I:C) for 1 h at 37 °C and then incubated with streptavidin agarose for another 2 h at 37 °C. The beads were washed three times with lysis buffer and analyzed by immunoblotting.
Coimmunoprecipitation and immunoblot analysis
Cells were lysed in 1 mL of NP-40 lysis buffer (20-mM Tris-HCl, pH 7.4–7.5, 150-mM NaCl, 1-mM EDTA, 1% NP-40, 10-μg/mL aprotinin, 10-μg/mL leupeptin, and 1-mM phenylmethylsulfonyl fluoride). The lysates were centrifuged at 12,000 rpm for 10 min at 4 °C. For each immunoprecipitation assay, the supernatant was incubated with 0.5 μg of the indicated antibody and 35 μL of a 50% slurry of GammaBind G Plus-Sepharose at 4 °C for 2 h. The beads were then washed three times with 1 mL of lysis buffer containing 500-mM NaCl. The bound proteins were separated by SDS-PAGE, and the associated proteins were analyzed by immunoblotting.
Poly(I:C) and LPS injection
Age- and sex-matched Zfyve1+/+ and Zfyve1−/− mice were injected i.p. with poly(I:C) (2 μg/g of body weight) plus D-galactosamine (0.25 mg/g of body weight) or LPS (10 μg/g). The survival of the injected mice was monitored every 0.5 h. Blood was collected 4 h after injection for ELISA of the cytokine levels.
CRISPR/Cas9 knockout
Genome engineering was performed using the CRISPR/Cas9 system.18,19 Double-stranded oligonucleotides corresponding to the target sequences were cloned into the lentiCRISPR v2 vector and cotransfected with packaging plasmids into 293 cells. Two days after transfection, the viruses were harvested and used to infect HT1080 or THP-1 cells. The infected cells were selected with puromycin (1 μg/mL) for at least 5 days. The following sequences were used to target human ZFYVE1 mRNA: #1: 5′- TATACACTCGGTTGTCATAC-3′ and #2: 5′- ACACTCGGTTGTCATACTGG-3′.
Flow cytometry
The spleens, thymi, and peripheral lymph nodes were obtained from Zfyve1+/+ and Zfyve1−/− mice, and single-cell suspensions were prepared. After the depletion of red blood cells by ammonium chloride, the cells were stained with the indicated antibodies for 30 min, followed by flow cytometry analysis.
Preparation of recombinant proteins
The cDNAs encoding ZFYVE1 (415-777), TLR3 (24-704), and cGAS were cloned into the pGEX-6p-1-GST plasmid. The plasmids were transformed into the E. coli BL21 strain. Expression of the GST fusion proteins was induced with 0.1-mM IPTG at 16 °C for 24 h. The recombinant proteins were purified with GST resins and eluted with elution buffer (PBS, 100-mM Tris-HCl, pH 8.8, 40-mM reduced glutathione).
Microscale thermophoresis technology (MST)
MST analysis was performed using a NanoTemper Monolith NT.115 instrument (NanoTemper Technologies GmbH). To determine the affinity between poly(I:C) and GST-ZFYVE1 (415-777) or GST-TLR3 (24-704), the proteins were fluorescently labeled with a Blue-NHS labeling kit according to the manufacturer’s instructions. Lysine residues in the tested proteins were labeled with a dye:protein molar ratio of 3. To determine the affinity between poly(I:C) and the GST-ZFYVE1 (415-777)/GST-TLR3 (24-704) complex, 20-nM Cy5-labeled GST-TLR3 (24-704) saturated with an amount of GST-ZFYVE1 (415-777) determined based on their Kd values was mixed with different concentrations of poly(I:C). To determine the affinity between the synthetic DNA HSV60 and ZFYVE1 (415-777) or cGAS, 20-nM Cy5-labeled HSV60 was mixed with different concentrations of proteins in PBS with 100-mM Tris-HCl, pH 8.8. To determine the affinity between HSV60 and the cGAS/ZFYVE1 (415-777) mixture, 20-nM Cy5-labeled HSV60 was mixed with different concentrations of GST-cGAS saturated with an amount of GST-ZFYVE1 (415-777) determined based on their Kd values. In these experiments, GST was used as a negative control. Samples were loaded into premium coated capillaries, and MST measurements were performed using 20% MST power and 40% LED power at 25 °C. Laser on and off times were 30 and 5 s, respectively. NanoTemper Analysis 1.2.20 software was used to fit the data and determine Kd values.33
Subcellular fractionation
To isolate mitochondrial, membrane, and cytosolic fractions, 293-TLR3 cells (2 × 107) were washed with PBS and lysed by dounce homogenization 40 times in 2 mL of homogenization buffer (Beyotime). The homogenate was centrifuged at 500 × g for 10 min. The supernatant (S5) was centrifuged at 5000 × g for 10 min to precipitate mitochondria (P5K). The supernatant from this step (S5K) was further centrifuged at 50,000 × g for 30 min to yield P50K, which contained the membrane fraction, and S50K, which mainly consisted of cytosol.
To isolate the nuclear fraction, 293-TLR3 cells (2 × 107) were washed with PBS and lysed by dounce homogenization 40 times in 1 mL of CER buffer (Applygen). The homogenate was centrifuged at 500 × g for 5 min, and the pellet containing the crude nuclei was then washed twice with 500 μL of NER buffer (Applygen).
Proteinase K protection assays
Proteinase K protection assays were performed as described.3 293-TLR3 cells (3 × 108) stably expressing HA-ZFYVE1-Flag were collected and washed twice with ice-cold PBS. The endosome/lysosome fractions were isolated from the cells by calcium precipitation and treated with proteinase K (2 μg/mL) or proteinase K (2 μg/mL) plus 0.2% Triton X-100 for 15 min at 55 °C or were left untreated. The samples were then mixed with 2× SDS loading buffer and analyzed by immunoblotting.
Statistical analysis
Unpaired Student’s t test was used for statistical analysis with Microsoft Excel and GraphPad Prism Software. For the mouse survival study, Kaplan–Meier survival curves were generated and analyzed by the log-rank test; P < 0.05 indicated significance.
Supplementary information
Acknowledgements
This work was supported by grants from the State Key R&D Program of China (2017YFA0505800, 2016YFA0502102 and 2018YFA0800700), the National Natural Science Foundation of China (31830024, 31630045, 31870870, 31800728, 31771555, and 31671418), the China Postdoctoral Science Foundation (2017M620334), the Fundamental Research Funds for the Central Universities (2042019kf0204), and the Natural Science Foundation of Hubei Province (2018CFA016).
Author contributions
H.-B.S. and X.Z. designed the study; X.Z., L.F., W.-H.X., X.W., and Y.-D.D. performed the experiments; H.-B.S., C.-Q.L., Y.Z., and X.Z. analyzed the data; and H.-B.S., C.-Q.L., and X.Z. wrote the paper.
Competing interests
The authors declare no conflict of interest.
Contributor Information
Cao-Qi Lei, Phone: +86-27-68753795, Email: caoqilei@whu.edu.cn.
Hong-Bing Shu, Email: shuh@whu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-019-0265-6) contains supplementary material.
References
- 1.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, et al. The RNA-binding protein Mex3B is a coreceptor of Toll-like receptor 3 in innate antiviral response. Cell Res. 2016;26:288–303. doi: 10.1038/cr.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsumoto M, et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 2003;171:3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell JK, Askins J, Hall PR, Davies DR, Segal DM. The dsRNA binding site of human Toll-like receptor 3. Proc. Natl. Acad. Sci. USA. 2006;103:8792–8797. doi: 10.1073/pnas.0603245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Bouteiller O, et al. Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J. Biol. Chem. 2005;280:38133–38145. doi: 10.1074/jbc.M507163200. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Liu L, Davies DR, Segal DM. Dimerization of Toll-like receptor 3 (TLR3) is required for ligand binding. J. Biol. Chem. 2010;285:36836–36841. doi: 10.1074/jbc.M110.167973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita M, et al. Epidermal growth factor receptor is essential for Toll-like receptor 3 signaling. Sci. Signal. 2012;5:ra50. doi: 10.1126/scisignal.2002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Cattaneo A, et al. Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc. Natl. Acad. Sci. USA. 2012;109:9053–9058. doi: 10.1073/pnas.1115091109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qi R, Singh D, Kao CC. Proteolytic processing regulates Toll-like receptor 3 stability and endosomal localization. J. Biol. Chem. 2012;287:32617–32629. doi: 10.1074/jbc.M112.387803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toscano F, et al. Cleaved/associated TLR3 represents the primary form of the signaling receptor. J. Immunol. 2013;190:764–773. doi: 10.4049/jimmunol.1202173. [DOI] [PubMed] [Google Scholar]
- 13.Zhu S, Wang G, Lei X, Flavell RA. Mex3B: a coreceptor to present dsRNA to TLR3. Cell Res. 2016;26:391–392. doi: 10.1038/cr.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnsen IB, et al. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J. 2006;25:3335–3346. doi: 10.1038/sj.emboj.7601222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MM, Shu HB. Multifaceted roles of TRIM38 in innate immune and inflammatory responses. Cell Mol. Immunol. 2017;14:331–338. doi: 10.1038/cmi.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo WW, Shu HB. Delicate regulation of the cGAS-MITA-mediated innate immune response. Cell Mol. Immunol. 2018;15:666–675. doi: 10.1038/cmi.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu YH, et al. WDFY1 mediates TLR3/4 signaling by recruiting TRIF. EMBO Rep. 2015;16:447–455. doi: 10.15252/embr.201439637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalem O, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridley SH, et al. FENS-1 and DFCP1 are FYVE domain-containing proteins with distinct functions in the endosomal and Golgi compartments. J. Cell Sci. 2001;114:3991–4000. doi: 10.1242/jcs.114.22.3991. [DOI] [PubMed] [Google Scholar]
- 21.Nanao T, Koike M, Yamaguchi J, Sasaki M, Uchiyama Y. Cellular localization and tissue distribution of endogenous DFCP1 protein. Biomed. Res. 2015;36:121–133. doi: 10.2220/biomedres.36.121. [DOI] [PubMed] [Google Scholar]
- 22.He J, et al. Membrane insertion of the FYVE domain is modulated by pH. Proteins. 2009;76:852–860. doi: 10.1002/prot.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung PC, Trinkle-Mulcahy L, Cohen P, Lucocq JM. Characterization of a novel phosphatidylinositol 3-phosphate-binding protein containing two FYVE fingers in tandem that is targeted to the Golgi. Biochem. J. 2001;355:113–121. doi: 10.1042/bj3550113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma R, Bharti K. Toll like receptor 3 and viral infections of nervous system. J. Neurol. Sci. 2017;372:40–48. doi: 10.1016/j.jns.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 25.Luo WW, et al. iRhom2 is essential for innate immunity to DNA viruses by mediating trafficking and stability of the adaptor STING. Nat. Immunol. 2016;17:1057–1066. doi: 10.1038/ni.3510. [DOI] [PubMed] [Google Scholar]
- 26.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Lian H, et al. The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA sensing and activation of the RIG-I-like receptors. Immunity. 2018;49:438–448. doi: 10.1016/j.immuni.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, et al. ZDHHC11 modulates innate immune response to DNA virus by mediating MITA-IRF3 association. Cell. Mol. Immunol. 2018;15:907–916. doi: 10.1038/cmi.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin H, et al. MARCH3 attenuates IL-1beta-triggered inflammation by mediating K48-linked polyubiquitination and degradation of IL-1RI. Proc. Natl. Acad. Sci. USA. 2018;115:12483–12488. doi: 10.1073/pnas.1806217115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang LQ, et al. IFITM3 inhibits virus-triggered induction of type I interferon by mediating autophagosome-dependent degradation of IRF3. Cell. Mol. Immunol. 2018;15:858–867. doi: 10.1038/cmi.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu MM, et al. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity. 2016;45:555–569. doi: 10.1016/j.immuni.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, et al. TRIM32-TAX1BP1-dependent selective autophagic degradation of TRIF negatively regulates TLR3/4-mediated innate immune responses. PLoS Pathog. 2017;13:e1006600. doi: 10.1371/journal.ppat.1006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian H, et al. ZCCHC3 is a co-sensor of cGAS for dsDNA recognition in innate immune response. Nat. Commun. 2018;9:3349. doi: 10.1038/s41467-018-05559-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.