Fig. 8. Etiology of Procedure Induced Retinal Lesions in Mice.
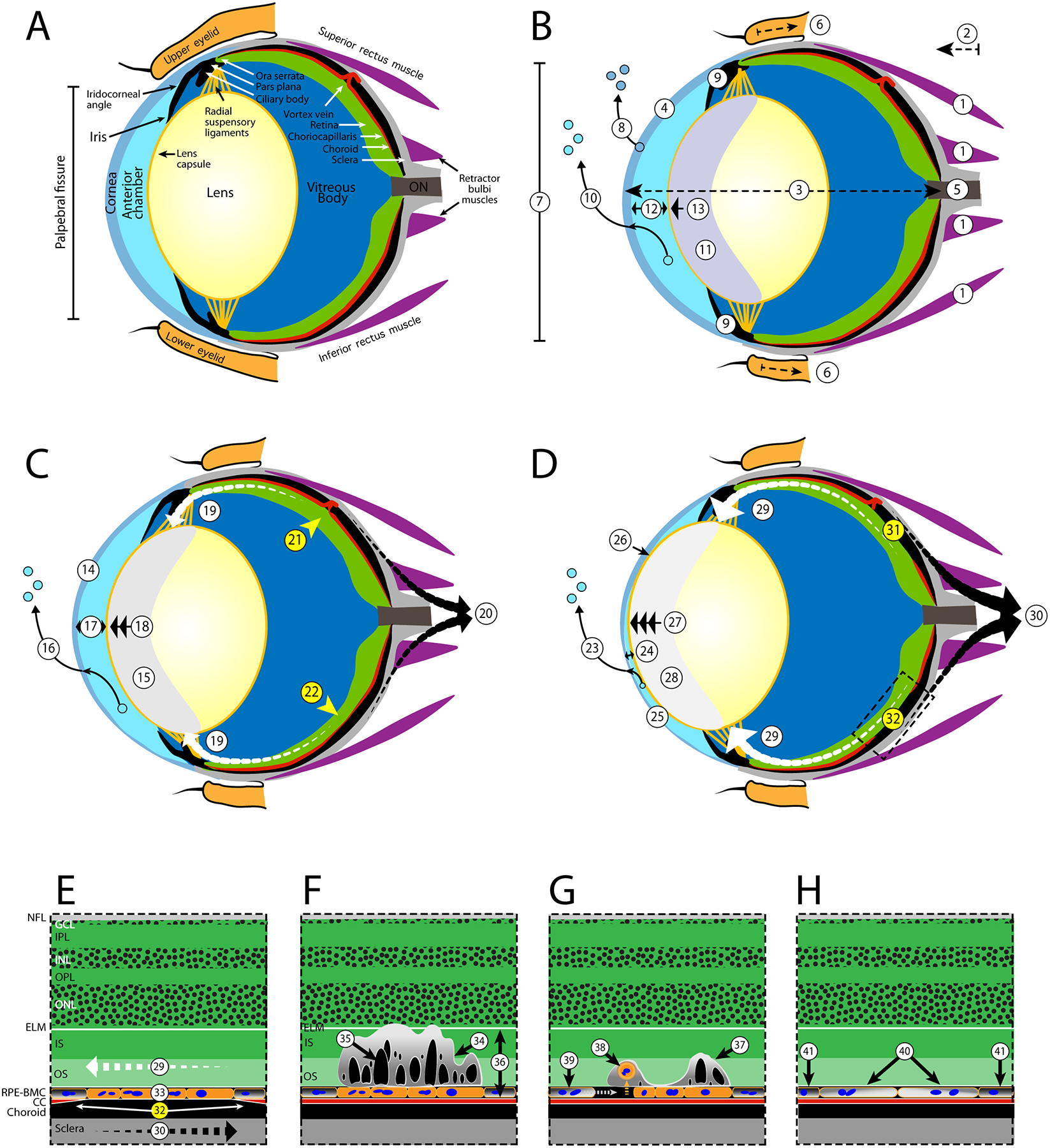
Suspected processes leading to mechanically induced ischemia (A-D) and the latent development of outer retinal lesions (E-G) in a normal mouse eye. For ischemia development, the changes are divided into three periods (B-D) that describe early (< 20 min), mid (20–40 min), and late (40 + min) time frames. For lesions, the earliest record of their presence at 3-days post-EPIP is demonstrated (E). Lesions do substantially regress over time as depicted by 14- and 80-days post-recovery illustrations (FG). A. Normal eye - Schematic of a normal adult mouse eye, modified from Remtulla and Hallett (1985) and Tkatchenko et al., (2010), detailing the anatomical regions and features suspected to be impacted post-EPIP. Note: the vortex vein is shown in the incorrect anatomical position for purposes of demonstrating stenosis and pinch-occlusion in the mid and late time phases, respectively. B. Early phase - General anesthesia and mydriasis induction with xylazine and phenylephrine result in adrenergic agonist activation and systemic changes that include smooth muscle relaxation, vasoconstriction, decreased perfusion, hypoxia, hypercapnia, bradycardia, respiratory acidosis, hypothermia, etc. (changes not depicted). Immediately post-EPIP, extraocular muscles become relaxed (1), causing exophthalmia (2), globe elongation (3), modified corneal curvature (4), increased load on the optic nerve (5), retraction of the eyelids and cessation of blink reflex (6), widening of the palpebral fissure (7), and rapid depletion of corneal tear film (8). Aqueous humor production and outflow via the ciliary bodies and Schlemm’s canal are reduced (9) followed by transcorneal loss of aqueous humor (10) and development of lens media opacity that persists until full recovery from sedation (11). A reduction in aqueous humor volume results in decreasing anterior chamber depth (12) followed by migration of the ocular lens (13) into the chamber. Extraocular pupillary appearance observed at this time has a faint bluish-hue due to lens media opacity formation (11). C. Mid phase Asymptotic limits for corneal desiccation and thinning (14) are reached followed by lens media opacity magnitude, area and integrated density (15). Extraocular pupillary appearance transitions from bluish-gray to grayish-white due (15). Sustained loss of aqueous humor via transcorneal evaporation (16) leads to additional reductions in anterior chamber volume/depth (17) and lens prolapse (18). Lens prolapse applies tension on the radial suspensory ligaments, Ciliary bodies, Pars Plana, Ora Serrata and RPE-BMC (19). Opposing forces between the RPE-BMC (19-white arrows) and the choroid (20-black arrows) induce an inner vs. outer laminar strain and impede blood perfusion via mechanically-induced stenosis of the vortex veins (21) and choriocapillaris (22). Lesion development probability approaches 50% during the mid-phase period. D. Late phase - Prolonged desiccation to the exposed eye assures that retinal lesions and other ocular complications are imminent. Evaporative fluid loss continues to deplete aqueous humor volume (23), further decreasing anterior chamber depth (24), resulting in a misshapen cornea (25), occasionally causing lens misalignment and resulting in the risk of adhesion to the corneal endothelium (26). A 3-to 4-fold change in lens prolapse (27) gives the pupil a bright white appearance (28) and applies increasing tension on the radial suspensory ligaments, the Ciliary bodies, Pars Plana, Ora Serrata and RPE-BMC (29). Estimated probability (~51–95%) for lesion development occurs from 39 to 55 min post-EPIP as increasing shear occurs between RPE-BMC (29-segmented tapered white arrows) and the choroid (30- segmented tapered black arrows) increases severity of the ischemic regions (31–32) that involve either pinch-occlusion of a vortex vein (31) or regional mechanical-induced stenosis of the choriocapillaris (32). E. Expanded FOV of Ischemia Affected Area - Opposing forces acting along the RPE-BMC (29) and choroid (30) mechanically collapse the choriocapillaris (32) causing an ischemic episode contributing to anaerobic stress to the RPE (33). Upon recovery from sedation, all various ocular systems aforementioned return to normal and the choriocapillaris undergoes reperfusion (not shown). Abbreviations: Nerve Fiber Layer (NFL), Ganglion Cell Layer (GCL), Inner Plexiform Layer (IPL), Inner Nuclear Layer (INL), Outer Plexiform Layer (OPL) Outer Nuclear Layer (ONL), External Limiting Membrane (ELM), Photoreceptor Inner (IS) and Outer Segments (OS), Retinal Pigment Epithelium & Bruch’s Membrane complex (RPE-BMC), and Choriocapillaris (CC). F. 3-Days Post-EPIP - Three days following recovery, prominent retinal lesions are viewable via fundus imaging and indicate areas that previously underwent ischemia-reperfusion injury post-EPIP. A cross-sectional view illustrates the characteristics of a lesion when observed at this time point using SD-OCT imaging. Lesions involve the IS and OS laminar structures of the photoreceptor layer and show some displacement of the ELM (34). Numerous subretinal cysts (35) are visible within the impacted lamina (34). RPE cells outside the ischemia-affected region are presumably normal (36), as outer retina lamina appear normal outside the affected regions. G. 14-Days Post-EPIP - Fourteen days following recovery, lesions show evidence of resolving and become more difficult to discern by fundus imaging. SD-OCT shows an area of involvement less pronounced than that observed at 3-days post-EPIP. However, a displaced photoreceptor layer containing sub-retinal features presents containing smaller and less numerous cysts (37). Histological evidence suggests that RPE cells encountered enough stress to become apoptotic and undergo detachment from Bruch’s membrane (38). Detached cells undergo phagocytosis (38) and removal followed by expansion of adjacent RPE cells to cover the exposed area (39). H. 80-Days Post-EPIP - Imaging evidence indicates that prominent outer retinal disruptions observed at 3-days post-EPIP are 99% resolved following 2.5 months of recovery. However, imaging data also suggest that the RPE remains compromised by the original insult and is morphologically different from adjacent, unaffected regions. Remaining or adjacent RPE cells, which underwent expansion to cover exposed areas of cell loss, exhibit altered melanosome or melanin granule pigment density (40) compared to normal RPE cells from unaffected regions (41) and suggest impaired melanogenesis or altered melanodistribution in RPE cells affected by the ischemia-reperfusion injury.
