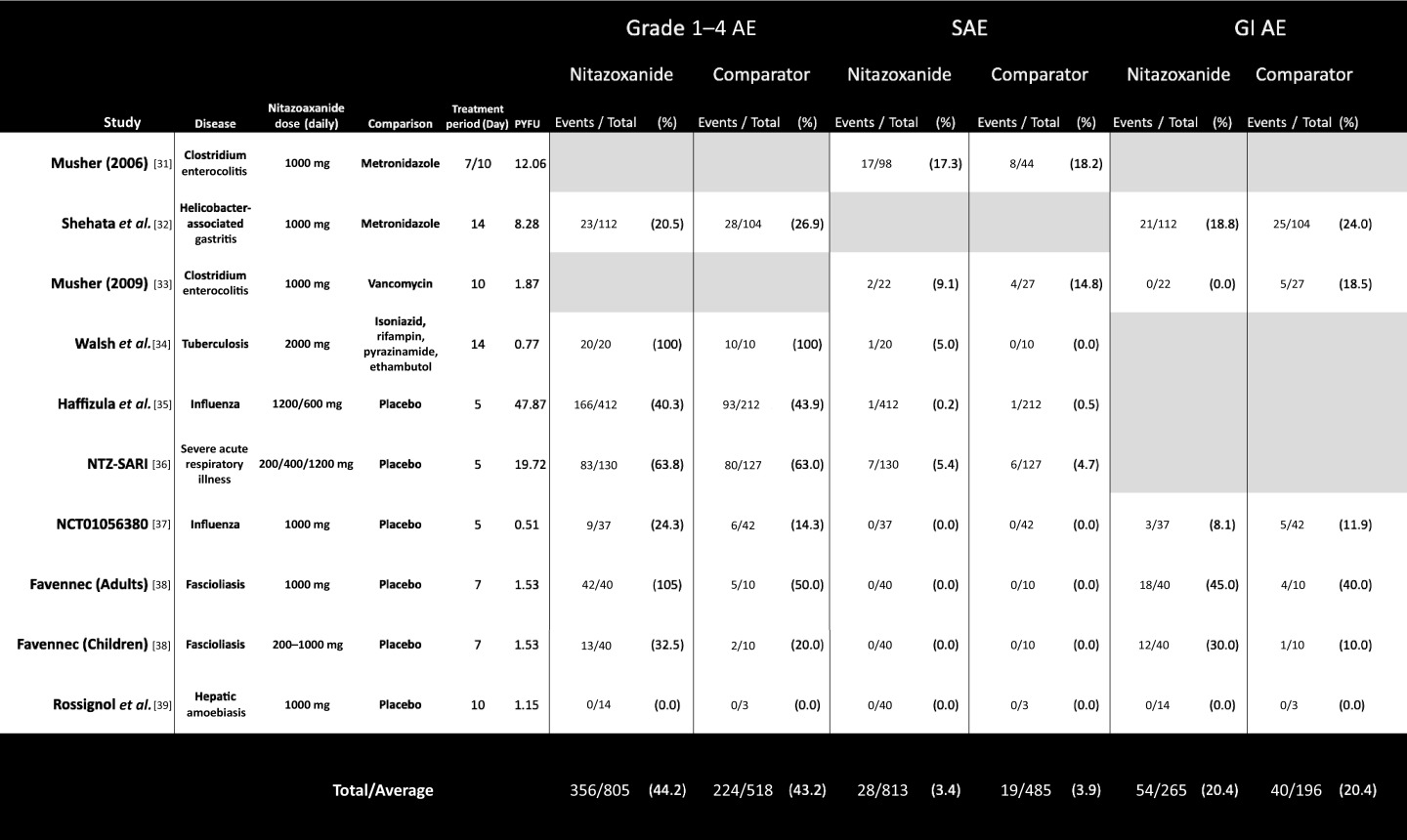
Table 1.
Summary of safety data extracted from the six phase 2 and 3 controlled studies with adverse event reporting. Extracted data for six reported safety endpoints shown for each included study
|
AE: adverse events; SAE: serious adverse events; GI: gastrointestinal.
