Table 3.
Safety data related to hepatic, renal or cardiovascular adverse effects of nitazoxanide, where reported across the nine included studies
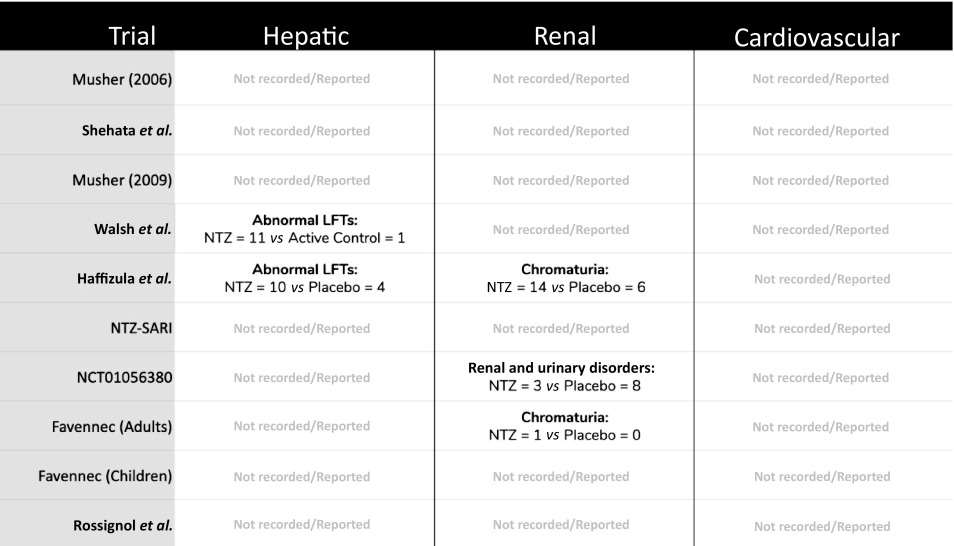
|
LFTs: liver function tests; NTZ: nitazoxanide; Hepatic: LFT or other relevant biochemical abnormality; Renal: any urinary or renal-related biochemical abnormality (creatinine, urea, electrolytes etc); Cardiovascular: (B-type natriuretic peptide, troponin, chest pain, QT prolongation, cardiac events etc).
