Figure 5. Liganding non-catalytic tyrosines for blockade of protein activity.
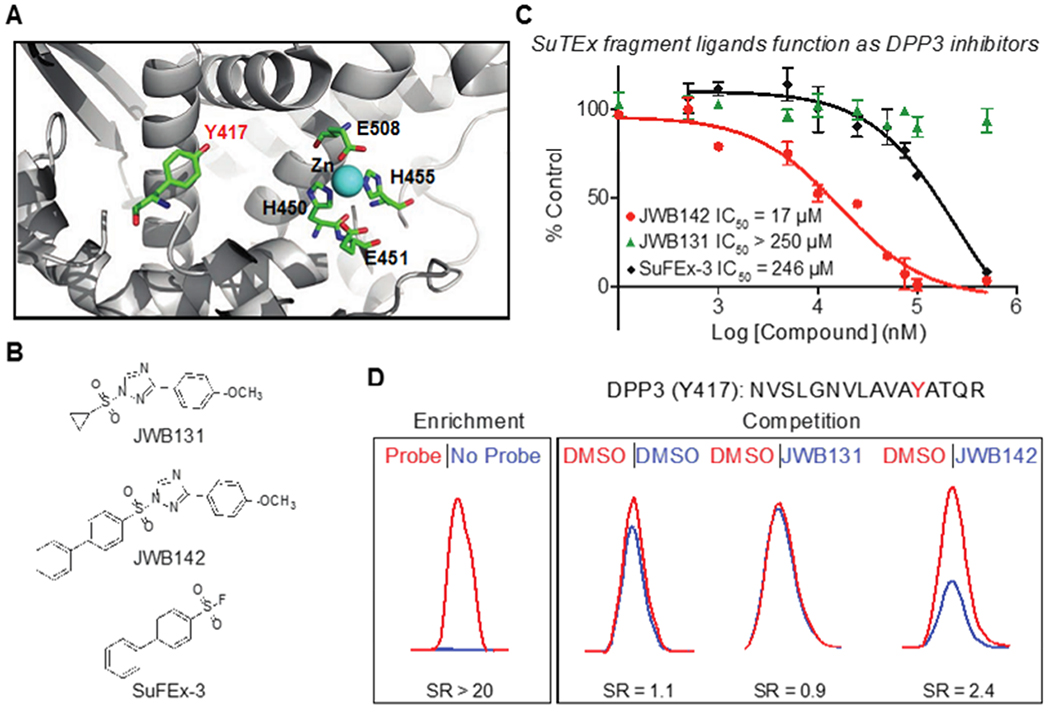
(A) Crystal structure of human DPP3 active site (PDB accession code 3FVY). The location of residues involved in zinc metal binding (H450, H455, E508), the catalytic glutamate (E451), and a non-catalytic tyrosine 417 (Y417) identified by SuTEx are highlighted. (B) Lead SuTEx fragments (JWB142) and negative control probe (JWB131) identified from a gel-based chemical proteomic screen against recombinant DPP3 proteomes (Supplementary Figure 7). (C) JWB142 but not JWB131 blocked catalytic activity of purified DPP3 in a concentration-dependent manner as measured by substrate assay: JWB142, IC50 = 17 μM, 95% confidence intervals: 11-27 μM. JWB142 showed >10-fold increase in inhibitory activity compared with the SuFEx counterpart: SuFEx-3, IC50 = 246 μM, 95% confidence intervals: 117-519 μM. Data are shown as mean ± s.e.m.; n = 3 biologically independent experiments. (D) DPP3 Y417 site is liganded (~50% blockade) by JWB142 but not JWB131 fragment as judged by quantitative chemical proteomic analysis of recombinant human DPP3-HEK293T soluble cell proteome. All data shown are representative of n = 2 biologically independent experiments.
