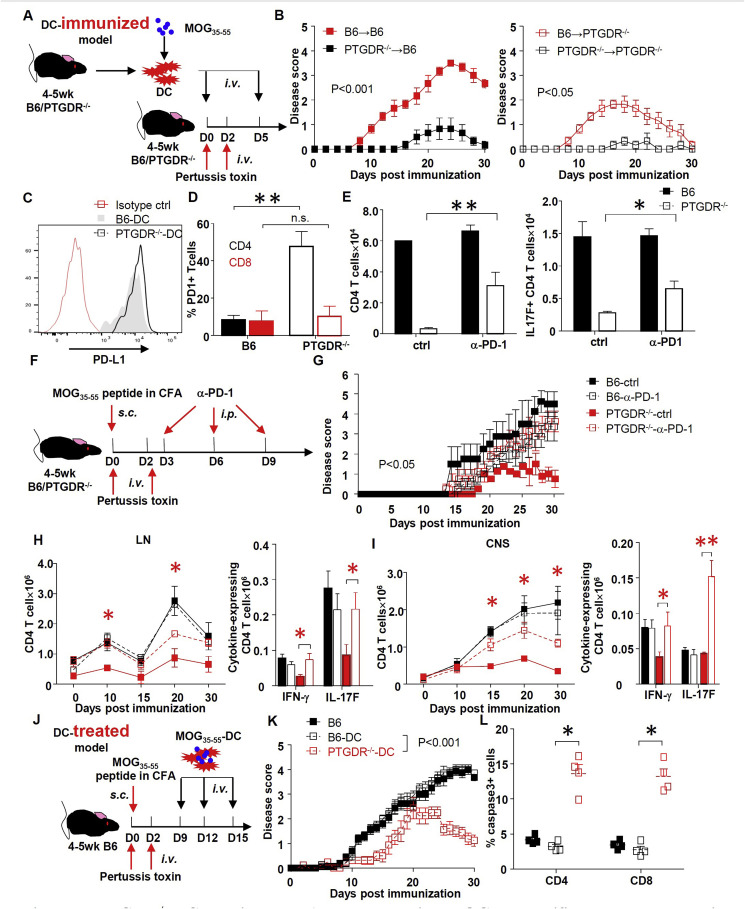
Fig. 6.
PTGDR−/−DC ameliorates EAE by decreasing MOG35-55-specific T cell response via enhanced PD-1 signaling. (A) Protocol for EAE induction using MOG35-55-DC immunization. (B) Disease scores of PTGDR−/− and B6 mice after DC immunization, n = 5. (C) Expression of PD-L1 on DCs harvested from LN of PTGDR−/− and B6 mice at day 10 post immunization. (D) Expression of PD-1 on CD4 and CD8 T cells harvested from LN of PTGDR−/− and B6 mice at day 10 post immunization, n = 4. (E) The number of total and MOG35-55-specific IL-17F-secreting CD4 T cells after treatment in vitro with α-PD-1 neutralizing antibody or isotype control (ctrl), n = 4. (F) Protocol for in vivo PD-1 blockade. (G) Disease score of immunized mice receiving α-PD-1 neutralizing antibody or isotype control, n = 5. (H and I) Dynamics of total CD4 T cell accumulation and cytokine expression by CD4 T cells in LNs (H) and CNS (I), after MOG35-55 peptide stimulation at day 10 post immunization (n = 4). *(red) difference between PTGDR−/−-ctrl and PTGDR−/−-α-PD-1 groups. (J) Protocol for MOG35-55-DC treatment of established EAE in B6 mice. (K) Disease scores of mice receiving DC-peptide treatment, n = 15. (L) Caspase-3 positive CD4 and CD8 T cells in LNs of recipients harvested on day 10 post immunization, n = 5. (B-E, G-I, K, L) Data are shown as mean ± SEM and are representative of 3 independent experiments. *p < 0.05, **p < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)