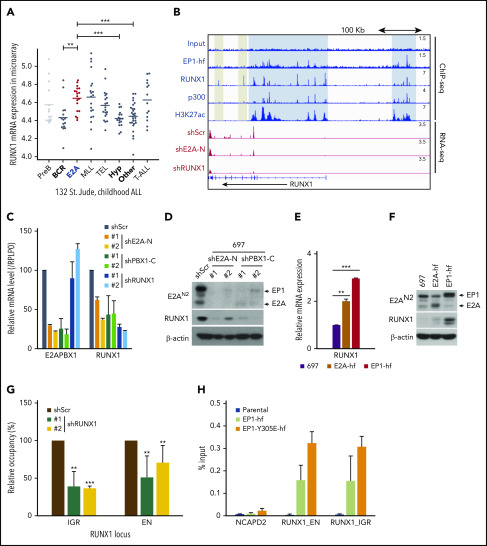
Figure 5.
E2A-PBX1 directly binds to and activates the RUNX1 gene. (A) RUNX1 is significantly upregulated in E2A-PBX1+ and MLL-rearranged B-ALL. The expression levels of RUNX1 in different subtypes of B-ALL patients are shown. Data are from St. Jude Children's Research Hospital, n = 132 B-ALL patients.59 Statistics by two-sided Wilcoxon test. **P < .01; ***P < .001. (B) RUNX1 is a direct target of E2A-PBX1. ChIP-seq profiles (upper) of indicated factors at the RUNX1 locus. Blue bars denote the colocalization of E2A-PBX1 (EP1-hf), RUNX1 and p300, and associated H3K27 acetylation; gray bars denote the lack of active marks in the RUNX1-binding regions. RNA-seq profiles (bottom) reveal the reduction of RUNX1 expression in the 697 cell line treated with shE2A-N and shRUNX1. (C) RT-qPCR assays showing the mRNA levels of E2A-PBX1 and RUNX1 in the 697 cell line treated with shScr, shE2A-N, shPBX1-C, or shRUNX1. (D) Immunoblot of E2A/E2A-PBX1 (with E2AN2 antibody) and RUNX1 in the 697 cell line treated with shScr, shE2A-N, or shPBX1-C. Actin was used as the loading control. (E-F) RUNX1 is upregulated by ectopic E2A-PBX1. RT-qPCR (E) and immunoblot (F) showing the expression of RUNX1 in the stable 697 cell line inducibly expressing E2A or E2A-PBX1. Total RNA or cell lysates were collected 24 hours’ postinduction. (G) ChIP-qPCR of E2A-PBX1 (HA) at upstream (EN) and intragenic (IGR) enhancers of the RUNX locus in the stable 697 cell line treated with shScr or shRUNX1. ChIP signals (y-axis) from 3 independent experiments were compared with control (Scramble) and are presented as mean ± SD. Statistics by Student t test. **P < .01; ***P < .001. (H) ChIP-qPCR of WT and Y305E E2A-PBX1 (HA) at enhancers of the RUNX1 locus in stable 697 cell lines. The NCAPD2 locus was used as a negative control for E2A-PBX1 binding. ChIP signals (y-axis) from 3 independent experiments are presented as mean ± SD.