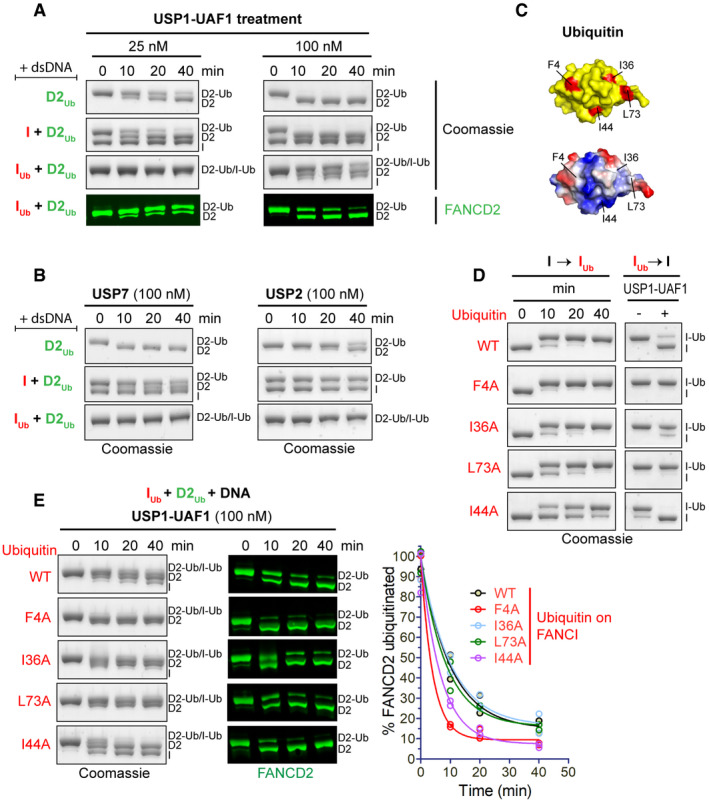
-
A
USP1‐UAF1 can efficiently deubiquitinate D2Ub in the presence or absence of FANCI (I), but not in the presence of ubiquitinated FANCI (IUb). Ubiquitinated FANCD2 (D2Ub) was mixed with a 50 base pair dsDNA and either His6‐TEV‐V5‐FANCI (I), ubiquitinated His6‐TEV‐V5‐FANCI (IUb) or no protein; protein–DNA mixes were subsequently incubated with USP1‐UAF1 (at 25 nM or 100 nM) for indicated time periods. Deubiquitination of D2Ub and IUb was assessed, following SDS–PAGE, by Coomassie staining of the gels, as well as by Western blotting of transferred blots with a specific FANCD2 antibody.
-
B
USP7 and USP2 can deubiquitinate D2Ub, but their activity towards D2Ub is greatly reduced in the presence of FANCI (I) or ubiquitinated FANCI (IUb). Reactions were set up as in (A), but with 100 nM USP7 or USP2. Deubiquitination was assessed, following SDS–PAGE, by Coomassie staining.
-
C
Location of the four hydrophobic residues mutated to alanine in the ubiquitin structure (PDB: 1ubq). Top: location of F4, I36, I44 and L73 (shown in red) in ubiquitin's surface (shown in yellow). Bottom: same as above but with ubiquitin's surface coloured according to charge (red: negative, blue: positive, white: no charge).
-
D
Time course of FANCI ubiquitination with various ubiquitin mutants and corresponding sensitivity/resistance to USP1‐UAF1‐mediated deubiquitination of resulting products. Deubiquitination reactions in the presence or absence of USP1‐UAF1 (100 nM) incubated for 30 min.
-
E
Time course of USP1‐UAF1‐mediated deubiquitination of IUbD2Ub‐DNA complexes consisting of D2Ub a 50 base pair dsDNA and IUb produced with various ubiquitin mutants or wild‐type (WT) ubiquitin. Progress of deubiquitination reaction was assessed following SDS–PAGE, by both Coomassie staining of the gels and Western blotting of transferred blots with a specific FANCD2 antibody. FANCI ubiquitination with indicated ubiquitin mutants (or WT) and subsequent deubiquitination of resulting IUbD2Ub‐DNA complexes were conducted twice; the residual FANCD2 ubiquitination, calculated from the FANCD2 blots for each time‐point, was plotted for each type of ubiquitin in the protein complex, and corresponding deubiquitination progress curves were fitted using a one‐phase decay model.