Figure 5. Model of how di‐monoubiquitination may shift the ID2 conformation to an enhanced DNA‐binding state.
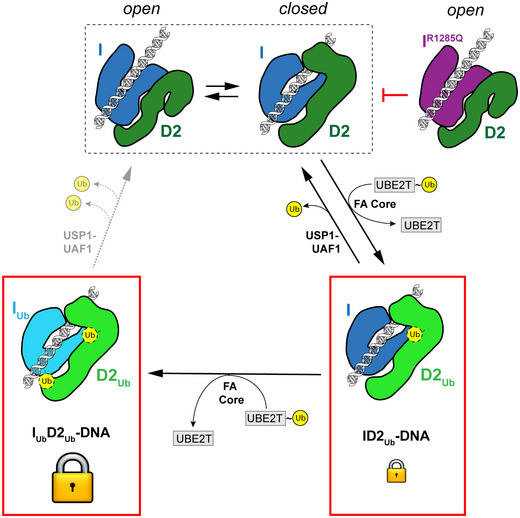
ID2 interaction with DNA is proposed to promote a dynamic equilibrium where ID2 can exist in both an open and a closed conformation. FANCD2 ubiquitination by Ube2T and the FA core complex, within a closed ID2 conformation status, can shift this equilibrium in favour of the closed conformation, with the action of USP1‐UAF1 counteracting such shift. When FANCI is also ubiquitinated by Ube2T and the FA core complex, both ubiquitins are largely resistant to USP1‐UAF1 deubiquitination, and thus, the doubly ubiquitinated ID2 can remain in the closed conformation. The latter conformation has a tighter DNA affinity and hence locks ID2 onto DNA. The R1285Q mutation on FANCI likely restricts formation of a closed ID2 conformation, which in turn negatively impacts on ID2 ubiquitination and locking onto DNA.
