Abstract
The intestinal epithelium serves as a dynamic barrier to the environment and integrates a variety of signals, including those from metabolites, commensal microbiota, immune responses and stressors upon ageing. The intestine is constantly challenged and requires a high renewal rate to replace damaged cells in order to maintain its barrier function. Essential for its renewal capacity are intestinal stem cells, which constantly give rise to progenitor cells that differentiate into the multiple cell types present in the epithelium. Here, we review the current state of research of how metabolism and ageing control intestinal stem cell function and epithelial homeostasis. We focus on recent insights gained from model organisms that indicate how changes in metabolic signalling during ageing are a major driver for the loss of stem cell plasticity and epithelial homeostasis, ultimately affecting the resilience of an organism and limiting its lifespan. We compare findings made in mouse and Drosophila and discuss differences and commonalities in the underlying signalling pathways and mechanisms in the context of ageing.
Keywords: ageing, epithelial barriers, intestinal homeostasis, metabolism, stem cells
Subject Categories: Metabolism, Molecular Biology of Disease, Regenerative Medicine
The intestinal epithelium plays important roles in nutrition, signaling and as a barrier to the environment. Intestinal stem cells are crucial to replace epithelial cells and to maintain epithelial homeostasis. This review discusses how aging and metabolism affect stem cell function and epithelial homeostasis in the mouse and fly intestine.
Introduction
The intestine is one of the most versatile epithelia in our body; it is involved in many physiological processes, including nutrient uptake as well as immune modulation and displays the interface to a complex commensal microbiome. Importantly, the intestine is also responsible for the absorption of nutrients throughout our lifetime. It serves as an important physical and chemical barrier to the environment, while also functioning as an integration site that responds to diverse physiological and pathophysiological stimuli. The stimuli include a wide range of metabolites, signals from the immune system and communication with the resident bacteria (Fig 1). Together, the crosstalk between these stimuli can dynamically impact the intestine in response to environmental or organismal challenges. Ageing poses an additional challenge for a constantly renewing epithelium and its protective function. The resulting functional consequences include adaptation of stem cell (SC) proliferation and differentiation, SC exhaustion, cellular senescence, immune and inflammatory responses as well as the reinforcement of epithelial barrier function (Fig 1). Importantly, these factors influence the epithelial homeostasis and barrier function of the intestine, and their aberrant regulation can lead to epithelial barrier defects and subsequent detrimental systemic effects on the entire organism (Nicoletti, 2015). Thus, identifying the mechanisms leading to robust epithelial function is of great importance and biomedical interest to counteract increased susceptibility to gastrointestinal disorders with age (Sovran et al, 2019) and to treat patients with metabolic syndromes (Thaiss et al, 2018).
Figure 1. The gut functions as a dynamic barrier to the environment with signal integration properties.
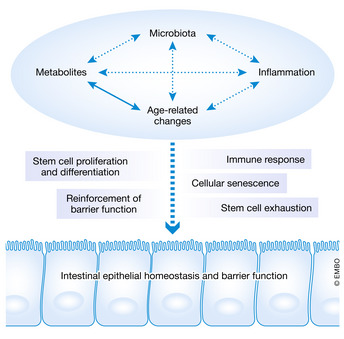
The gut functions as a chemical and physical barrier to the environment and serves as an integration site to respond to diverse intrinsic as well as extrinsic stimuli. These stimuli display a wide range of metabolites, age‐related changes, microbiota as well as inflammation‐associated processes. The resulting functional consequences include adaption in stem cell and proliferation, immune responses, reinforcement of the barrier function, cellular senescence as well as stem cell exhaustion. Functional consequences constantly affect the epithelial homeostasis and subsequently the barrier function of the intestine, eventually leading to epithelial barrier defects.
The fruit fly Drosophila melanogaster and mice are widely used genetic model systems to study human diseases (Aitman et al, 2011; Lehner, 2013). Over the last decades, Drosophila and mice have contributed important insights into diverse biological processes in the intestine. This review focuses on intestinal homeostasis, metabolism and ageing, highlighting both similarities and differences between vertebrates and invertebrates. In addition, we discuss the potential consequences of these interactions on the epithelial barrier and, thus, organismal effects.
Principal concepts of intestinal homeostasis, metabolism and ageing
Intestinal homeostasis
Epithelial homeostasis is dependent on a balance between intestinal stem cell (ISC) self‐renewal, progenitor differentiation, cell shedding and apoptosis (see Fig 2 for a schematic of fly and mouse intestine). In this context, the capacity of ISCs to decide between self‐renewal and differentiation allows for dynamic response and remodelling of the epithelium in response to external stimuli. Both, the Drosophila and mouse intestine, undergo rapid cell turnover, with a self‐renewal rate of 3–5 days in the murine intestine (Cheng & Leblond, 1974). In the murine intestine, the main driver for this high proliferation is Wnt ligands, mainly secreted by Paneth cells (PCs) and the underlying mesenchyme, with both Wnt sources seemingly functionally redundant for the maintenance of intestinal homeostasis (Sato et al, 2011; Kabiri et al, 2014; Valenta et al, 2016). In Drosophila, the visceral muscles similarly produce the Wnt ligand wingless (wg), which has also been implicated in ISC maintenance (Lin et al, 2008). In Drosophila, as well as in the mouse intestine, tissue homeostasis is based on a neutral competition between symmetrically dividing SCs (Snippert et al, 2010; de Navascués et al, 2012). In differentiating progenitors, Notch signalling is responsible for suppressing the secretory lineage and promoting enterocyte (EC) differentiation in both, the Drosophila and mouse systems (Milano et al, 2004; Fre et al, 2005; Guo & Ohlstein, 2015). Upon damage, or in response to external cues, the equilibrium is shifted towards self‐renewal or proliferation, as will be discussed later. In summary, homeostasis of the intestinal epithelium is key for its functionality and maintenance of the epithelial barrier, which is essential for organismal health.
Figure 2. Schematic representation of the anatomy of Drosophila and mouse intestine.
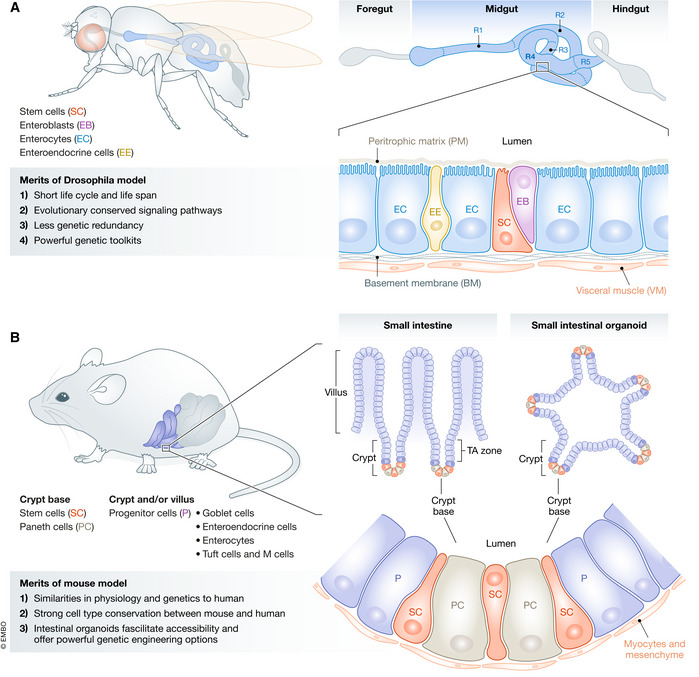
(A) The Drosophila digestive tract is composed of foregut, midgut and hindgut. The cell types in the adult intestine include: stem cells (SC), enteroblasts (EB), enterocytes (EC) and enteroendocrine cells (EE). The intestinal epithelium is surrounded by visceral muscles and peritrophic membrane that separates the intestinal cells from bacteria presented in the lumen. (B) The epithelium of the mouse small intestine is structured into the crypt region, the transit amplifying (TA) region and the villus region. Stem cells (SC) of the intestine are located in the crypts and are surrounded by Paneth cells (PC), which provide essential growth factors to the SC and are part of the stem cell niche. Transit amplifying cells that have left the crypt region are pushed upwards to the villi and driven towards differentiation in the different cell types of the intestinal epithelium, including goblet cells (GC), enteroendocrine cells (EE), tuft cells, M cells and enterocytes (EC). The intestinal epithelium is underlined by a muscle layer and mesenchyme.
Intestinal metabolism
Caloric restriction (CR) has been proposed to promote longevity in a wide range of organisms (Fontana & Partridge, 2015), and current efforts aim to shed light on the molecular mechanisms underlying this organismal effect. The intestinal epithelium is in direct contact with nutrients and metabolites, representing a first site where CR, or other diet regimes, could impact on the organism. In recent years, new insights have been gained into how different nutritional states can influence ISC function and thereby epithelial homeostasis. Two different modes of response can be distinguished: a direct influence of metabolites on ISC function by modulating signalling pathways and an indirect response of ISCs on changes in the dietary status to remodel the cellular composition of the epithelium. Moreover, the response can be ISC‐intrinsic or mediated via other epithelial cell types, such as neighbouring Paneth cells in mice or ECs in Drosophila.
Intestinal ageing
In a rapidly renewing tissue, ISCs are particularly challenged to keep up with the speed of proliferation for self‐renewal and progenitor differentiation (Cheng & Leblond, 1974). Additionally, over the lifespan of the organism, ISCs suffer from increased replicative age, including the accumulation of cellular and DNA damage that can occur during every round of replication (Liu & Rando, 2011). Thus, ISCs are highly prone to stem cell exhaustion, an integrative hallmark of ageing (López‐Otín et al, 2013). This ageing phenotype is characterized by a reduced self‐renewal capacity of ISCs, a diminished tissue self‐repair function after damage and misbalanced progenitor differentiation. These consequences influence the epithelial homeostasis and barrier function, which is frequently observed in the elderly as leaky gut syndrome (Kiefer & Ali‐Akbarian, 2004). In addition to cell‐dependent stem cell exhaustion, metabolism can also impact on intestinal homeostasis. Since the basal metabolic rate and nutrient usage change during organismal ageing (Henry, 2000; Houtkooper et al, 2011), metabolism might have additional effects on the ageing tissue, causing changes in ISC function and progenitor differentiation, as well as on how cells respond to external and internal stimuli.
Drosophila and mouse, two widely used genetic model systems, show common and distinct features that are essential for intestinal homeostasis, providing a ground for cross‐species investigation to unravel evolutionarily conserved mechanisms and the fundamental concepts of intestinal homeostasis. Nearly all genes mentioned in this review have homologs in the human genome (Tables 1 and 2), indicating that conserved mechanisms between mouse and fly are likewise relevant for human intestinal homeostasis and ageing.
Table 1.
Drosophila genes discussed in the review with their predicted homolog in mouse and human (Homology score based on flybase.org algorithm)
| Drosophila gene | Mouse homolog | Homology score | Human homolog | Homology score |
|---|---|---|---|---|
| Metabolism | ||||
| Ahcy | Gm4737 | +++ | AHCY | +++ |
| AhcyL1 | AhcyL1 | +++ | AHCYL1 | +++ |
| AhcyL2 | AhcyL2 | +++ | AHCYL2 | +++ |
| Crtc | Crtc1 | +++ | CRTC1 | +++ |
| mGluR | Grm3 | +++ | GRM3 | +++ |
| CanB | Ppp3r1 | +++ | PPP3R1 | +++ |
| Drp1 | Dmn1 l | +++ | DNM1L | +++ |
| Gcn2 | Eif2ak4 | +++ | EIF2AK4 | +++ |
| park | Prkn | +++ | PRKN | +++ |
| Pgam5 | Pgam5 | +++ | PGAM5 | +++ |
| Pgant4 | Galnt10 | +++ | GALNT10 | +++ |
| Pink1 | Pink1 | +++ | PINK1 | +++ |
| Signalling | ||||
| AMPKα | Prkaa2 | +++ | PRKAA2 | +++ |
| Ilp3 | Igf1 | + | INS | + |
| InR | Insr | +++ | INSR | +++ |
| Pi3K21B | Pik3r3 | +++ | PIK3R3 | +++ |
| srl | Pprc1 | +++ | PPRC1 | +++ |
| Tor | Mtor | +++ | MTOR | +++ |
| Thor | Eif4ebp1 | +++ | EIF4EBP1 | +++ |
| Dl | Dll1 | +++ | DLL1 | +++ |
| N | Notch1 | +++ | NOTCH1 | +++ |
| msn | Tnik | +++ | TNIK | +++ |
| wts | Lats2 | +++ | LATS1 | +++ |
| yki | Yap1 | +++ | YAP1 | +++ |
| Upd3 | Il‐6 | N/A | IL‐6 | N/A |
| hop | Jak2 | +++ | JAK2 | +++ |
| Stat92E | Stat5b | +++ | STAT5B | +++ |
| bsk/JNK | Mapk8 | +++ | MAPK10 | +++ |
| Myc | Myc | ++ | MYC | ++ |
| Other functions | ||||
| Brf | Brf1 | +++ | BRF1 | +++ |
| eEF2 | Eef2 | +++ | EEF2 | +++ |
| FoxK | Foxk1 | +++ | FOXK2 | +++ |
| Ire1 | Ern1 | +++ | ERN1 | +++ |
| Xbp1 | Xbp1 | +++ | XBP1 | +++ |
| Atg1 | Ulk1 | +++ | ULK1 | +++ |
| bbg | Pdzd2 | ++ | PDZD2 | + |
| Col4a1 | Col4a1 | +++ | COL4A6 | +++ |
| crc | ATF4 | ++ | ATF4 | ++ |
| Piwi | Piwil1 | +++ | PIWIL3 | +++ |
| prom | Prom1 | +++ | PROM2 | +++ |
| Tk | Tac1 | + | TAC1 | + |
| Tg | Tgm1 | +++ | TGM1 | +++ |
| Wdr62 | Wdr62 | +++ | WDR62 | +++ |
The table is also included in the Appendix, where all genes are linked to their respective NCBI gene entry. Homology score: 1–2: +, 3–5: ++, 6–15: +++.
Table 2.
Mouse genes discussed in the review with their predicted homolog in Drosophila and human (Homology score based on flybase.org algorithm)
| Mouse gene | Drosophila homolog | Homology score | Human homolog | Homology score |
|---|---|---|---|---|
| Metabolism | ||||
| Stk11 (Lkb1) | Lkb1 | +++ | STK11 | +++ |
| Mpc1 | Mpc1 | +++ | MPC1 | +++ |
| Pdk4 | Pdk | +++ | PDK4 | +++ |
| Cpt1a | whd | +++ | CPT1A | +++ |
| Ppard | Eip75B | ++ | PPARD | +++ |
| Ppara | Eip75B | ++ | PPARA | +++ |
| Sirt1 | Sirt1 | +++ | SIRT1 | +++ |
| Hmgcs2 | HMGS | +++ | HMGCS2 | +++ |
| Signalling | ||||
| Wnt3 | wg | + | WNT3 | +++ |
| Lgr5 | rk | +++ | LGR5 | +++ |
| Ctnnb1 | arm | +++ | CTNNB1 | +++ |
| Notum | Notum | +++ | NOTUM | +++ |
| Notch1 | N | +++ | NOTCH1 | +++ |
| Atoh1 | cato | +++ | ATOH1 | +++ |
| Sox9 | Sox100B | ++ | SOX9 | +++ |
| Mapk14 (p38) | p38b | +++ | MAPK14 | +++ |
| Map2k6 (MKK6) | lic | +++ | MAP2K6 | +++ |
| Trp53 | p53 | ++ | TP53 | +++ |
The table is also included in the Appendix, where all genes are linked to their respective NCBI gene entry. Homology score: 1–2: +, 3–5: ++, 6–15: +++.
Intestinal physiology in Drosophila and mouse
The mammalian and Drosophila intestines share fundamental similarities, such as food digestion, absorption, immune defence and host–microbe symbiosis (Marianes & Spradling, 2013; Dutta et al, 2015). Additionally, the intestine of Drosophila and mice houses stem cells that utilize similar mechanisms to ensure homeostatic renewal of tissue and injury‐induced regenerative responses. These mechanisms include the activity of highly conserved signalling pathways in the regulation of stem cell functions. For example, Notch controls ISC maintenance and EC differentiation (Fre et al, 2005; Stanger et al, 2005; Guo & Ohlstein, 2015; Beumer & Clevers, 2016), epidermal growth factor (EGF) signalling activates ISCs to proliferate (Buchon et al, 2010; Biteau & Jasper, 2011; Jiang et al, 2011; Jiang & Edgar, 2012; Jin et al, 2015; Smith et al, 2016), Wnt/wingless signalling governs ISC maintenance as well as proliferation (Lin et al, 2008; de Lau et al, 2011; Cordero et al, 2012; Clevers, 2013; Guo et al, 2016), and BMP/Dpp signalling restricts ISC proliferation and promotes cell differentiation (Haramis et al, 2004; He et al, 2004; Guo et al, 2013; Zhou et al, 2015; Ma et al, 2019). In Drosophila, ISC proliferation and differentiation is additionally regulated by Unpaired/JAK/STAT signalling (Buchon et al, 2009; Jiang et al, 2009; Cordero et al, 2012; Zhai et al, 2017). The Drosophila intestinal epithelium is devoid of mesenchyme and several specialized cell types that are present in the mouse intestine, such as Paneth cells, goblet cells (GCs) and Tuft cells (Fig 2) (Zwick et al, 2018). While murine intestinal stem cells rely on niche factors (Wnt, Notch, EGF) provided by the mesenchyme and Paneth cells, the Drosophila intestine relies on visceral muscles (VMs) and enteroblasts (EBs) to produce these niche factors for ISC proliferation and differentiation to maintain epithelial homeostasis (Lin et al, 2008; de Lau et al, 2011; Cordero et al, 2012; Zhou et al, 2015; Gehart & Clevers, 2019). In both systems, ECs are the most abundant epithelial cell type and play important roles in nutrient absorption and immune homeostasis (Peterson & Artis, 2014).
The intestinal epithelium of both species can be repaired after injury. Studies in Drosophila suggest that differentiated ECs represent a source signal to direct ISC division for tissue turnover (Jiang et al, 2009; Liang et al, 2017). A recent study revealed that p38 signalling is activated in ECs to integrate multiple types of stress via a Nox‐Ask1‐MKK3‐p38 axis. This EC‐dependent response mediates ISC proliferation and intestinal regeneration (Patel et al, 2019). Moreover, the Hippo pathway has been identified to play a central role during intestinal regeneration in Drosophila and mice (Hong et al, 2016). In the mouse intestine, Yap, the transcriptional regulator of the Hippo pathway, is dispensable for intestinal homeostasis but required for the regeneration of the Lgr5+ ISC pool after damage (Gregorieff et al, 2015). In a recent work, single‐cell analysis after irradiation revealed a quiescent intestinal cell type, which is activated after injury in a Yap‐dependent manner. This cell type was termed revival stem cell and was shown to be required to replenish the ISC pool and restoration of intestinal function (Ayyaz et al, 2019). Besides the Hippo pathway, several other signalling pathways are involved in intestinal regeneration (Beumer & Clevers, 2016), such as Wnt signalling. Here, a conserved mechanism between Drosophila and mice was identified by Perea et al (2017), who showed that the Ret receptor tyrosine kinase is required for stem cell proliferation by regulating Wnt signalling. Accordingly, the Ras effector molecules RAL GTPases have also been shown to play a conserved role in promoting Wnt activity, which is important for ISC maintenance and regeneration (Johansson et al, 2019). These fundamental overlaps in cell type function and actively involved signalling pathways during intestinal homeostasis and regeneration highlight the importance of using both, Drosophila and mouse models, to understand intestinal physiology and to guide the development of new clinical approaches.
Intestinal functions in Drosophila
The anatomy of the Drosophila intestine
The adult Drosophila intestine is divided into three distinct regions: the foregut, midgut and hindgut. The midgut represents the endodermal counterpart of the adult mouse intestine and is the main focus of this review. The midgut is organized as a tube, composed of an epithelial monolayer, and is separated from the intestinal lumen by a peritrophic matrix, composed of chitin polymers and glycoproteins (Lehane, 1997) to protect itself from bacteria present in the lumen. The intestine is surrounded by a basement membrane and visceral muscles (VMs) (Miguel‐Aliaga et al, 2018) (Fig 2A). The midgut consists of four main cell types, namely SCs, postmitotic immature enteroblasts (EBs), which differentiate into functional absorptive enterocytes (ECs), and secretory enteroendocrine cells (EECs) (Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006; Jiang & Edgar, 2012) (Fig 2A). ISCs adhere via integrins to the VM, which, together with EBs and ECs, provide niche factors to the ISCs (Lin et al, 2008; Cordero et al, 2012). The merits of the Drosophila model for intestinal research are its short life cycle, evolutionarily conserved signalling pathways with human and mice (Neves et al, 2015), a low level of genetic redundancy and powerful genetic tools to manipulate gene function, using inducible and cell type‐specific approaches.
Intestinal homeostasis and metabolism
Since ISCs were identified in Drosophila, the intestine has been intensively used as a model system to investigate the interplay between metabolism, immune activity and regenerative responses during homeostasis (Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006). In response to metabolism and nutrient availability, adult Drosophila restricts intestinal growth during food scarcity in order to survive in a rapidly changing environment. Additionally, flies have developed an adaptive mechanism to direct symmetric division of stem cells to accelerate intestinal growth when food is abundant (O'Brien et al, 2011). Furthermore, studies have uncovered a role for the fragile X mental retardation protein/Lin‐28/insulin receptor (InR) axis in sensing nutrients and inducing stem cell expansion, symmetric division and intestinal proliferation (Chen et al, 2015; Luhur & Sokol, 2015; Luhur et al, 2017).
The role of dietary inputs in the regulation of ISC activity and intestinal epithelial turnover has recently started to be explored. For example, S‐adenosyl‐methionine (SAM) from dietary methionine acts as a critical molecule to regulate stem cell division in the midgut by controlling protein synthesis via a unique diphthamide modification on eukaryotic elongation factor 2 (Obata et al, 2018). SAM also regulates the expression of the JAK/STAT ligand Unpaired 3 (Upd3) in ECs, which is known to induce stem cell proliferation upon refeeding (Fig 3A) (Obata et al, 2018). Moreover, the abundance of food triggers SCs to employ the hexosamine biosynthesis pathway (HBP). Elevated HBP levels then induce a Warburg effect‐like metabolic switch and InR signalling activity to enhance ISC division and intestinal growth (Mattila et al, 2018) (Fig 3A). Another example of dynamic stem cell response to available metabolites/nutrients is dietary L‐glutamate, which activates the metabotropic glutamate receptor and causes high cytosolic Ca2+ to induce SC division and intestinal growth by regulating calcineurin (CaN) and CREB‐related transcriptional co‐activator (Crtc) (Deng et al, 2015) (Fig 3A). These data highlight the intestine as a dynamic tissue that adjusts SC activity for organ growth in response to food availability.
Figure 3. The interplay between metabolism, homeostasis and ageing in Drosophila intestine.
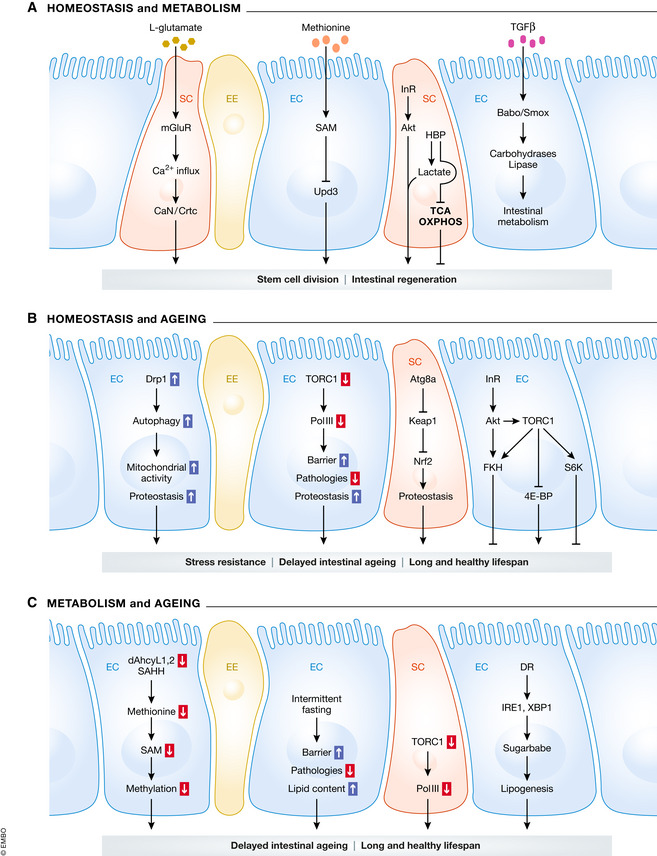
(A) Intestinal local signals control stem cell division and metabolism to maintain tissue homeostasis. Specifically, metabolic and signalling influxes influence downstream pathways in various cell types to regulate stem cell activity. (B) The pathways involved in autophagy, growth and metabolism improve intestinal function during ageing and extends animal lifespan. (C) The effects of diet restriction on intestinal barrier function and ageing. Blue arrows indicate an upregulation, and red arrows indicate a downregulation.
Intestinal cell populations communicate with each other to cooperatively support tissue regeneration and to regulate metabolic homeostasis (Miguel‐Aliaga et al, 2018). For instance, the muscle‐derived insulin‐like 3 peptide (dIlp3) activates InR signalling in stem cells, which drives diet‐stimulated intestinal growth (O'Brien et al, 2011). However, this intestinal SC–muscle interaction is severely impaired upon loss of tachykinin (Tk)‐ or Tk‐producing EECs, suggesting an important role of EECs in diet‐stimulated stem cell division and tissue growth (Amcheslavsky et al, 2014; Scopelliti et al, 2019). Indeed, EECs are required for nutrient sensing, and a subset of Dh31‐ and Tk‐expressing EECs are activated in the presence of proteins and amino acids (Park et al, 2016). These observations highlight the importance of SC‐EECs and SC‐muscle crosstalk in nutrient sensing, metabolic adaptation and organ growth. In addition to these mentioned examples, the communication between intestinal cells may influence other aspects of intestinal homeostasis, such as ageing, immunity and microbiota, which requires further investigation.
Enteroendocrine cells are known to produce hormones in response to external stimuli, such as nutrients, and to regulate local and systemic metabolism. Studies uncovered that EECs control intestinal and systemic lipid homeostasis, eventually affecting animals’ food intake, locomotor activity and lifespan (Song et al, 2014; Kohyama‐Koganeya et al, 2015, 2017; Takeda et al, 2018). In addition, dietary lipids modulate Delta ligand and Notch extracellular domain stability to guide EEC differentiation (Obniski et al, 2018). Interestingly, ISCs rely primarily on lipid metabolism, more specifically lipolysis, to serve as energy for their maintenance and survival (Singh et al, 2016). These data demonstrate an important role for EEC in linking dietary lipid or lipid metabolism with stem cell activity and intestinal growth (Amcheslavsky et al, 2014; Singh et al, 2016; Obniski et al, 2018).
In addition to lipid metabolism, other nutrients can influence intestinal growth. For example, yeast ingestion triggers the destabilization of the Misshapen and Warts proteins to allow Yorkie‐dependent intestinal growth (Li et al, 2018). Also, dietary sugar stimulates transforming growth factor beta (TGFβ) signalling in the intestine to regulate local and systemic metabolism (Chng et al, 2014; Song et al, 2017; Song et al, 2017) (Fig 3A). It was reported that pyruvate metabolism in enterocytes regulates stem cell proliferation by a canonical non‐autonomous activation of JAK/STAT signalling (Wisidagama & Thummel, 2019). A more recent study demonstrates Hodor as a metal sensor in the intestine that controls dietary zinc preference and impacts on food intake and insulin signalling for systemic growth (Redhai et al, 2020). These findings indicate that dietary nutrients (proteins, lipids and sugars) influence developmental signalling pathways to control intestinal metabolism and growth. Importantly, the majority of these effects influence the ISCs indirectly and rather mediated via other intestinal cell types.
Intestinal homeostasis and ageing
Ageing is an extremely complex and multifactorial process characterized by a time‐dependent functional decline. Increasing evidence indicates that intestinal regenerative capacity has a profound impact on animal lifespan (López‐Otín et al, 2013). In Drosophila, for instance, a mild reduction in ISC proliferation is associated with extended lifespan; however, strong inhibition of ISC proliferation disrupts intestinal regeneration and reduces lifespan (Biteau et al, 2010). On a mechanistic level, a study showed that the activation of c‐Jun N‐terminal kinase (JNK) stress response signalling caused homeostatic imbalance and intestinal dysplasia in aged intestines by accumulation of inappropriately differentiated stem cell daughter cells (Biteau et al, 2008). Increasing evidence indicates that improving mitochondrial biogenesis and proteostasis and limiting endoplasmic reticulum (ER) stress in ISCs can delay stem cell ageing (Rera et al, 2011; Wang et al, 2014, 2015; Rodriguez‐Fernandez et al, 2019). For example, overexpression of Drosophila peroxisome proliferator‐activated receptor gamma co‐activator 1 (PGC‐1) homologue (spargel) in ISCs increases mitochondrial activity, delays ageing‐related intestinal barrier dysfunction and extends lifespan in flies. Additionally, somatic SCs coordinate cell cycle arrest with protein clearance to prevent proteostasis failure in ageing flies (Rodriguez‐Fernandez et al, 2019) (Fig 3B).
In the aged intestine, homologous recombination during mitosis causes loss of heterozygosity in SCs, in turn leading to genetic mosaicism. Likewise, age‐related somatic deletion of DNA sequences and large structural rearrangement often cause gene inactivation (Siudeja et al, 2015). These genomic modifications of tumour suppressor genes, such as Notch, in SCs can result in tumour growth (Siudeja et al, 2015). Additionally, the age‐related heterochromatin instability in ECs induces genomic stress, JNK signalling and EC cell death, eventually causing non‐autonomous intestinal stem cell ageing (Jeon et al, 2018). Therefore, a range of cellular intrinsic cues, including DNA rearrangements and genomic instability, influences ISC ageing. Interestingly, during the functional loss of stem cells in ageing flies, ECs can dedifferentiate into ISCs through amitosis with tissue demand (Lucchetta & Ohlstein, 2017), indicating that the intestine utilizes amitosis as a mechanism to regenerate SCs to restore homeostasis. In addition, the argonaute protein family member Piwi is critical for ensuring heterochromatin maintenance, to suppress retrotransposon activation and prevent DNA damage. Loss of Piwi causes reduced SC activity and loss of their progenies by apoptosis, indicating that the genome integrity is essential for the maintenance and function of aged SCs (Sousa‐Victor et al, 2017). The same group also reported that restoring JNK/Wdr62/Kif1 governed mitotic spindle orientation in ISCs improves ageing‐related intestinal barrier function and extends lifespan (Hu & Jasper, 2019). Moreover, Filer et al, described an important link between target of rapamycin kinase complex 1 (TORC1) and RNA polymerase Pol III during ageing. Reducing Pol III activity specifically in ISCs extends the lifespan of flies by reducing aged‐related gut dysfunction (Filer et al, 2017) (Fig 3B). Collectively, improved mitochondrial activity, restored spindle orientation, reduced stress signalling, limited ER stresses, decreased TORC1/Pol III activity, preserved proteostasis and genomic stability in stem cells can promote intestinal homeostasis, reduce epithelial barrier dysfunction and extend animal lifespan (Biteau et al, 2008; Rera et al, 2011; Wang et al, 2014, 2015; Filer et al, 2017; Hu & Jasper, 2019; Rodriguez‐Fernandez et al, 2019).
The autophagy system also plays an important role in ageing (Escobar et al, 2019). Autophagy is a conserved mechanism that sequesters components of the cytoplasm into autophagosomes and further delivers them to lysosomes for degradation and macromolecule recycling. Autophagy plays important roles during development and disease by catabolizing intracellular components to maintain energy homeostasis (Hansen et al, 2018). In the context of ageing, intestinal‐specific AMP‐activated protein kinase (AMPK)/autophagy‐related gene 1 (Atg1) activation leads to reduced insulin‐like peptide levels in the brain and increased autophagy in both, the brain and intestine, slows systemic ageing and extends animal lifespan (Ulgherait et al, 2014). Moreover, the specific TOR inhibitor, rapamycin, induces autophagy in the intestine, reducing SC activity and delaying intestinal ageing in Drosophila (Fan et al, 2015). ISCs employ autophagy to eliminate the accumulation of cells with DNA damage and cell cycle arrest during ageing (Garschall et al, 2017; Nagy et al, 2018) (Fig 3B). ISCs with reduced lipolysis initiate JNK‐dependent autophagy and recycling by nearby differentiated cells (Singh et al, 2016). Recent studies revealed that impaired autophagy‐related pathway results in DNA damage, mitochondrial dysfunction (Rana et al, 2017) (Fig 3B), activation of mitogen‐activated protein kinase (MAPK) signalling and intestinal hyperplasia (Zhang et al, 2019) as well as stem cell misdifferentiation and short lifespan (Guo et al, 2019). These studies suggest that autophagy contributes to DNA damage clearance, recycling of unfit cells, restricting stem cell activity, slowing down tissue ageing and animal lifespan extension.
Inducing mitophagy, the selective clearance of mitochondria by autophagy, has been proposed to also have beneficial effects on organismal fitness, for instance, by improving mitochondrial unfolded protein response and proteolytic homeostasis (Borch et al, 2017), preventing mitochondrial damage (Koehler et al, 2017) and mitochondrial DNA mutations as well as enhancing stem cell activity (Hansen et al, 2018; Kauppila et al, 2018). NAD+ level has been shown to be decreased in the flies with pre‐ageing conditions, while supplementation of NAD+ precursors restores mitophagy, stem cell activity and extends fly lifespan, suggesting that metabolic intervention prevents stem cell exhaustion or ageing, which can benefit systemic ageing (Fang et al, 2019). Autophagy seems to play an essential role in ageing by eliminating unfit cells and improving mitochondrial activity and protein turnover, thereby preventing stem cell and systemic ageing. However, the underlying mechanisms are not completely understood. Given these findings, autophagy plays complex roles in maintaining intestinal homeostasis and ageing, and further detailed investigations are needed to address how the multistep autophagy processes can influence ageing.
Intestinal metabolism and ageing
Metabolic changes that occur during ageing include mitochondrial function, insulin sensitivity, metabolite shuttling and utilization and are influenced by genetic and environmental variation (Finkel, 2015). In several model organisms, including worms, flies and mice, calorie restriction (CR) has been shown to promote longevity (Tatar et al, 2014; Finkel, 2015; Kapahi et al, 2017). In Drosophila, dietary restriction induces a metabolic switch towards increased triglyceride usage and protects against age‐related pathologies, leading to lifespan extension (Mair et al, 2003; Piper & Bartke, 2008). Mechanistically, IRE1/XBP1 ER stress signalling and its downstream effector sugarbabe (Sug) are involved in triglyceride synthesis and their accumulation in ECs (Fig 3C). This mechanism mediates tissue metabolic adaptation that allows for dietary restriction‐induced lifespan extension (Luis et al, 2016) (Fig 3C). In response to dietary restriction of amino acids, the amino acid deprivation‐activated kinases GCN2 and ATF4 transcriptionally regulate the translational inhibitor 4E‐BP to mediate lifespan extension (Kang et al, 2017). In addition, intermittent fasting in early life has been shown to increase the lifespan and healthspan of flies with increased resistance to starvation, oxidative and xenobiotic stress and improved age‐associated gut pathologies and barrier function, which are considered as anti‐ageing phenotypes (Catterson et al, 2018) (Fig 3C). Dietary restriction also leads to dMyc upregulation with enhanced enterocyte cellular fitness and improved intestinal barrier function (Akagi et al, 2018). Notably, this enhanced enterocyte cellular fitness was sufficient to prevent intestinal ageing and increase the life expectancy of flies (Akagi et al, 2018). The intestine of female flies maintains more highly active SCs and is more resistant to external challenges than that of male flies, but also shows increased defects during ageing. Moreover, studies have shown that dietary restriction also prevents ageing‐related female gut pathologies and improves the healthspan of females more than it does those of male flies (Regan et al, 2016; Catterson et al, 2018). Taken together, dietary restriction induces transcriptional and post‐transcriptional changes on genes involved in metabolic adaptation, for example, facilitating lipogenesis and lipid usage that delays age onset intestinal pathologies and increases organismal lifespan.
Methionine is an essential amino acid in animals, and methionine restriction extends the lifespan of multiple model organisms such as Caenorhabditis elegans, rat, mouse and Drosophila (Orentreich et al, 1993; Sun et al, 2009; Cabreiro et al, 2013; Lee et al, 2014; Obata & Miura, 2015; Bárcena et al, 2018; Parkhitko et al, 2019). In addition, methionine serves as the precursor for the classical methyl donor SAM for methyltransferase activity, which is converted to S‐adenosyl‐homocysteine (SAH) (Obata & Miura, 2015; Parkhitko et al, 2016; Zhang, 2018). The alteration in SAM levels causes changes in epigenetic regulation, lipid metabolism and protein function (Lu & Mato, 2012). Interestingly, Obata & Miura (2015) reported that the systemic level of SAM is increased in aged flies. Overexpression of glycine N‐methyltransferase (Gnmt) enhances SAM catabolism, which suppresses the age‐dependent upregulation of SAM levels, resulting in lifespan extension (Obata & Miura, 2015). In addition, dietary restriction and reduced insulin signalling impede the age‐related SAM increase (Obata & Miura, 2015). Another study demonstrated that methionine metabolism changes dramatically during ageing and that SAH levels are increased in aged flies. An in vivo RNAi screen targeting methionine pathway components revealed that the Drosophila homologues SAH hydrolase Ahcy, dAhcyL1 and dAhcyL2 are involved in the regulation of lifespan (Parkhitko et al, 2016) (Fig 3C). Reprogramming of methionine metabolism by inhibiting these SAH hydrolases in Drosophila or specifically in the brain and intestine suppresses age‐related SAH increases and leads to lifespan extension (Parkhitko et al, 2016). In summary, increased SAM/SAH levels are associated with animal lifespan, and the regulation of SAM/SAH levels may be an effective strategy for lifespan extension.
In the context of amino acids, a low supply of optimized protein has been found to increase early life fitness (Piper et al, 2017). This enhanced dietary amino acid quality is beneficial for animal growth and reproduction without comprising lifespan (Piper et al, 2017). A systematic transcriptional analysis of Drosophila tissues identified conserved GATA family transcription factors that regulate the effect of dietary amino acids on lifespan in response to dietary restriction (Dobson et al, 2018). Interestingly, restriction of branched‐chain amino acids (BCAAs) also results in lipid accumulation, stress resistance and improved age‐associated gut pathology with extended lifespan (Juricic et al, 2020). These results demonstrate the importance of limiting dietary amino acid supply for lifespan extension, but the molecular details are still lacking.
In addition to amino acid availability and functionality, insulin activity also plays a central role in metabolism during ageing. In general, lowered insulin activity often results in long‐lived animals (Tain et al, 2017). Studies also showed that fat body‐specific reduction in protein translation and induction of proteasomal activity in the intestine contribute to the extended lifespan in insulin mutant flies (Essers et al, 2016; Tain et al, 2017). Accordingly, overexpression of the proteasome β5 subunit increases proteasome‐related chymotrypsin‐like activity, reduces ubiquitinated protein aggregates and increases the lifespan of adult flies (Nguyen et al, 2019). Intestinal‐specific upregulation of the FoxA transcription factor homologue fork head (FKH) improves gut barrier function in aged flies and extends lifespan (Bolukbasi et al, 2017). Mechanistically, FKH is phosphorylated by Akt and TOR, and phosphorylated FKH is required for rapamycin and the reduced insulin signalling‐related anti‐ageing effects (Bolukbasi et al, 2017) (Fig 3B).
Intestinal functions in mice
The anatomy of the mouse intestine
The intestine of the mouse, as in humans, can be separated into the small and large intestine, which includes the colon. In this review, we focus on the small intestine as it has been the prime focus of studies and is the functional equivalent to the Drosophila midgut. In contrast to the Drosophila midgut, the epithelial monolayer of the small intestine in mice is organized in crypt–villus units (Fig 2B). The villi are finger‐like protrusions that reach into the intestinal lumen to increase the absorptive surface, while crypts (also called crypts of Lieberkühn) are deep invaginations that house the ISCs (Bjerknes & Cheng, 1999; Barker et al, 2007) at their base (Fig 2B). These units are covered by secreted mucus towards the lumen and basally underlined by visceral muscle and mesenchyme (Fig 2B). Along the crypt–villus axis, seven different cell types can be identified (van der Flier & Clevers, 2009): the crypt base harbours the ISCs, intermingled with Paneth cells, which provide niche signals as well as bactericidal products. As cells are pushed from the stem cell niche and ascend the so‐called transit amplifying (TA) zone, they proliferate and differentiate. Differentiated cell types include secretory cells of the intestine, such as GCs and EECs, which secrete mucus and hormones, respectively, and can be found along the entire crypt–villus axis. Absorptive ECs display the most abundant cell type and are located along the villi. Tuft cells and microfold (M) cells are comparatively rare cell types in the small intestine and are involved in the immune response. Advantages of the mouse as a model for intestinal research are the high physiological and genetic similarity to humans (Nguyen et al, 2015), strong cell type conservation (Neves et al, 2015) and the possibility to generate intestinal organoids, which are long‐term 3‐dimensional cell cultures (Sato et al, 2009) (Fig 2B). Intestinal organoids markedly facilitate the experimental accessibility of the intestinal epithelium and stem cell niche and allow for versatile genetic engineering (Koo et al, 2012). The generation of organoids displays a stem cell‐driven process that reflects ISC function in vivo (Sato et al, 2009) and allows direct assessment of ISC regenerative capacity by measuring the organoid formation efficiency from a single stem cell or crypts in culture.
Intestinal homeostasis and metabolism
Over the last years, emerging data have shown that in addition to the well‐known regulatory signalling pathways, such as Notch and Wnt (Vermeulen & Snippert, 2014), energy metabolism likewise contributes to ISC function and epithelial homeostasis. In this context, the intestinal epithelium is influenced by intrinsic cellular metabolic state as well as by extrinsic cues, such as metabolites and nutritional states. ISCs have been proposed to integrate the metabolic status of an organism and respond dynamically to changes in the availability of metabolites and dietary status, such as CR and fasting (Yilmaz et al, 2012; Igarashi & Guarente, 2016; Mihaylova et al, 2018), ketogenic and glucose‐supplemented diet (Cheng et al, 2019), high‐fat diet (Beyaz et al, 2016) or cholesterol‐rich diet (Wang et al, 2018).
In addition to responding to the nutritional status, ISCs and Paneth cells themselves exhibit different metabolic states, which can drive proliferation and differentiation of intestinal cells (Rodríguez‐Colman et al, 2017; Schell et al, 2017). ISCs show high mitochondrial activity (OXPHOS) compared to Paneth cells, which are in turn characterized by pronounced glycolysis (Rodríguez‐Colman et al, 2017) (Fig 4A). These metabolic profiles directly influence ISC function, as inhibition of mitochondrial activity in ISCs as well as inhibition of glycolysis in Paneth cells leads to diminished organoid formation, which has been used as an assay for ISC function. Moreover, the metabolic compartmentalization of OXPHOS and glycolysis in ISCs and Paneth cells, respectively, appears to be an inherent feature of the stem cell niche. Paneth cells produce high levels of lactate during glycolysis, which they provide to ISCs to fuel their OXPHOS (Rodríguez‐Colman et al, 2017). Therefore, lactate serves as another niche signal secreted by the PCs, such as Wnts (Gregorieff, 2005), which allows Paneth cells to support ISCs in sensing the metabolic state and controlling differentiation versus proliferation in response to metabolic changes (Fig 4A).
Figure 4. The interplay between metabolism, homeostasis and ageing in the mouse intestine.
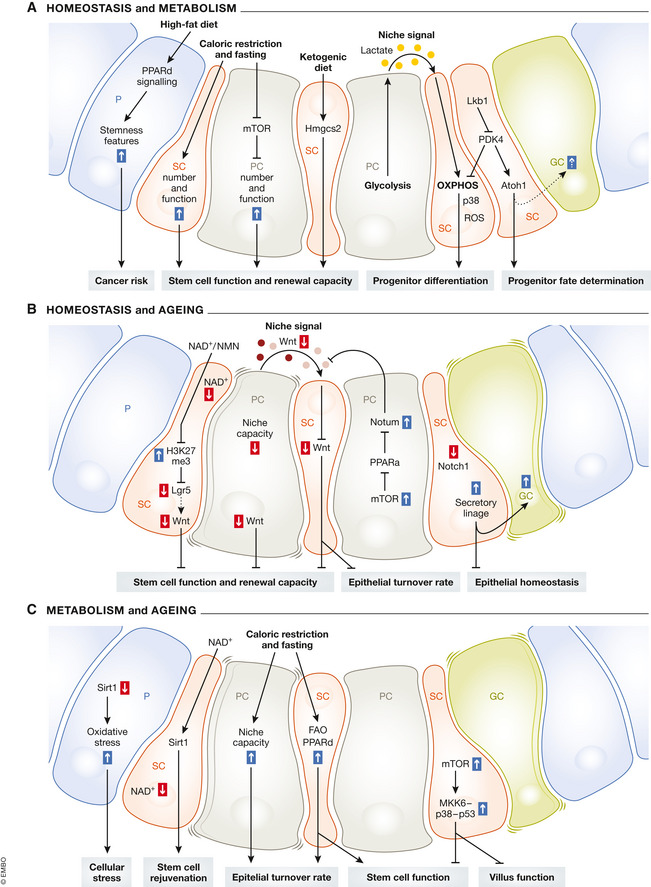
(A) Extrinsic metabolic changes, such as dietary interventions, and intrinsic metabolic states influence the epithelial homeostasis of the intestine. The response of the epithelium to metabolic changes includes stem cell function and renewal capacity, progenitor differentiation and fate determination as well as increased cancer risk. (B) Effects of ageing on the epithelial homeostasis in the mouse intestine include diminished stem cell function and renewal capacity as well as impaired epithelial turnover rate and homeostasis. (C) The effects of metabolites and metabolic changes on ageing‐related phenotypes include induced cellular stress, stem cell rejuvenation, improved epithelial turnover rate and stem cell function as well as impaired villus and stem cell function. Blue arrows indicate an upregulation, red arrows indicate a downregulation, dashed lines indicate suggested biological function, and solid lines indicate shown biological function.
Intestinal stem cells rely on mitochondrial activity to regulate their metabolic rate for the cell fate decision between proliferation and differentiation. On a mechanistic level, mitochondrial activation in ISCs drives differentiation into distinct intestinal cell types, a process that involves p38 (Mapk14) activity and mitochondrial ROS signalling (Fig 4A) (Rodríguez‐Colman et al, 2017). In this context, blocking oxidative pyruvate metabolism in the mitochondria of ISCs by deleting the mitochondrial pyruvate carrier Mpc1 increases stem cell proliferation. This results in an expansion of the ISC compartment at the expense of cellular differentiation in the intestine of mice as well as Drosophila (Schell et al, 2017). Thus, mitochondrial OXPHOS facilitates differentiation of intestinal stem cells, while its inhibition leads to an increase in the stem cell pool. This metabolic linkage enables the intestinal epithelium to respond dynamically to changes in metabolic states and adjust the balance between stem cell proliferation versus differentiation. Recently, a link between metabolism and cell fate determination was identified. Here, loss of Lkb1, a bioenergetic sensor, in ISCs resulted in a boost of secretory cell types (Gao et al, 2020). This differentiation bias towards the secretory lineage was independent of Notch signalling, which is instructive during homeostasis for the cell fate decision between absorptive and secretory cell types (Fre et al, 2005; Pellegrinet et al, 2011). Instead, the authors identified that during homeostasis, LKB1 inhibits Atoh1, the master regulator for the secretory lineage (Yang, 2001), via inhibition of the pyruvate dehydrogenase kinase PDK4 (Fig 4A). PDK4 is an inhibitor of pyruvate dehydrogenase and thereby OXPHOS. Upon loss of Lkb1 in ISCs, OXPHOS is deregulated and Atoh1 levels are elevated, resulting in an increased progenitor differentiation into secretory cells (Gao et al, 2020). Thus, the metabolic state of intestinal stem cells is involved in both, the regulation of proliferation versus differentiation and cell fate determination of progenitor cells. Yet, it remains to be elucidated how OXPHOS levels are timely fine‐tuned in stem cells to first direct towards differentiation, which requires high OXPHOS levels, and subsequently for cell fate decisions, which might require different OXPHOS levels. Moreover, it will be interesting to assess how different dietary regimes influence the metabolic state of ISCs and to what extent intrinsic and extrinsic responses to nutrition are linked and feed into each other.
As observed for the metabolic state, the nutrition status of the organism can also have a direct impact on ISCs or an indirect impact mediated via Paneth cells and niche‐induced changes. For example, CR leads to an increase in ISC and Paneth cell numbers, as well as enhanced ISC function (Fig 4A), identified by improved organoid formation (Yilmaz et al, 2012; Igarashi & Guarente, 2016). This response to CR involves mechanistic target of rapamycin kinase (mTOR) complex 1 (mTORC1) signalling in Paneth cells and induces a shift of equilibrium towards ISC renewal at the expense of differentiation, for example, into absorptive cells in response to reduced food intake (Fig 4A) (Yilmaz et al, 2012; Igarashi & Guarente, 2016). These findings further support the hypothesis that Paneth cells have a supportive role in sensing nutritional availability and contribute to a dynamic response by modulating ISC function. In summary, ISCs and Paneth cells communicate in response to CR and mTOR signalling is centrally involved in transmitting this response.
In comparison to CR, short‐term fasting results in a direct stem cell‐intrinsic response, where they switch to fatty acid metabolism (Mihaylova et al, 2018). This effect was identified by co‐culturing fasted ISCs with ad libitum Paneth cells and vice versa to generate intestinal organoids. Thereby fasting alone improved ISC capacity, indicating that the fasting‐mediated response in ISCs is highly dynamic and independent of Paneth cells, in contrast to the CR‐mediated response (Yilmaz et al, 2012; Igarashi & Guarente, 2016; Mihaylova et al, 2018). Mechanistically, this metabolic change is facilitated by the activation of peroxisome proliferator‐activated receptor delta (PPARd) signalling and mediated by CPT1A. Moreover, the positive fasting effect in ISCs was mimicked by turning on fatty acid oxidation (FAO) metabolism via drug‐induced activation of PPARd (Fig 4A), revealing options for future drug treatments (Mihaylova et al, 2018).
In contrast to caloric reduction, increased caloric intake by a high‐fat diet (HFD) has diverse effects on ISCs and Paneth cells, increasing the number of ISCs while reducing the number of Paneth cells (Beyaz et al, 2016). Elevated stem cell numbers are in line with another study (Mah et al, 2014), which shows that HFD leads to a higher rate of stem cell divisions and may thereby contribute to an increased risk of intestinal tumours upon HFD. On a mechanistic level, HFD activates PPARd signalling, which activates a restricted ß‐catenin programme in ISCs as well as non‐ISC (Lgr5‐GFPlow) progenitor cells (Fig 4A). As a result, ISCs become less dependent on Paneth cells, for example during organoid formation. Likewise, progenitor cells showed stemness features (Fig 4A) and were able to form organoids independently. Thus, HFD induces stem cell divisions and thereby potentiates the number of cells of origin for tumours. Moreover, non‐ISC progenitor cells acquire stemness potential and may also contribute to potential sites of tumour initiation (Beyaz et al, 2016).
Alongside CR, fasting and high‐fat diet, cholesterol availability was recently shown to affect and modulate ISC function and to promote ISC proliferation (Wang et al, 2018). Moreover, a ketogenic diet was identified to increase ISC number and function, which was counteracted by a glucose‐supplemented diet (Cheng et al, 2019). Mechanistically, ISCs express high levels of Hmgcs2, an enzyme that produces ketone bodies, including beta‐hydroxybutyrate (ßOHB), which in turn inhibits histone deacetylases to sustain Notch signalling and mediate intestinal homeostasis (Cheng et al, 2019) (Fig 4A). In summary, these studies demonstrate that diet and metabolism have a direct impact on ISC function and influence intestinal homeostasis.
Moreover, the regenerative capacity of the intestinal epithelium can be influenced by CR. Hereby CR‐mediated inhibition of mTORC1 preserves the activity of reserve stem cells, which allows for an improved epithelial regeneration after injury (Yousefi et al, 2018). Accordingly, genetic mTOR disruption severely affects the regeneration capacity after irradiation (Sampson et al, 2016).
Intestinal homeostasis and ageing
The effect of ageing in the intestinal epithelium was initially studied by descriptive analyses, which identified changes in the intestinal architecture, such as altered villi structure and cell type composition. Moreover, these age‐related changes were linked to deteriorated ISC activity (Martin et al, 1998), indicating the importance of ISC function for tissue homeostasis. On a functional level, exhaustion of aged ISCs is displayed in vivo as measured by a reduced tissue self‐repair capacity after damage and a slower turnover of the epithelium in aged mice (Nalapareddy et al, 2017). Moreover, aged ISCs have limited capacity to form organoid cultures in vitro (Moorefield et al, 2017; Nalapareddy et al, 2017) as well as attenuated differentiation potential estimated by crypt number per organoid (Nalapareddy et al, 2017; Cui et al, 2019; Pentinmikko et al, 2019). On a mechanistic level, stem cell exhaustion can be caused by increased DNA damage. ISCs acquire a high replicative age due the fast renewal rate of the epithelium, which is further potentiated by a reduced DNA damage response of aged ISCs (Watanabe et al, 2019).
In terms of changes in stem cell number upon ageing, various studies have identified either an ISC expansion and hyperproliferation (Moorefield et al, 2017), unchanged ISC numbers (Kozar et al, 2013; Nalapareddy et al, 2017) or a reduction (Mihaylova et al, 2018; Pentinmikko et al, 2019). One reason for these contradictory observations might be due to the different reporter lines used in these studies such as EGFP‐Sox9 (Moorefield et al, 2017) and Lgr5‐EGFP‐IRES‐CreERT2 (Nalapareddy et al, 2017; Mihaylova et al, 2018), as well as the continuous‐labelling approach used by one study (Kozar et al, 2013). Thus, further investigations are needed to explore the mechanism of ISC exhaustion. The use of currently emerging single‐cell techniques may be one way to address these questions.
The characterization of epithelial homeostasis in aged intestines revealed an increase in Paneth cells (Nalapareddy et al, 2017; Mihaylova et al, 2018; Pentinmikko et al, 2019) and goblet cells (Moorefield et al, 2017; Nalapareddy et al, 2017; Igarashi et al, 2019), which indicates a change in the differentiation potential of ISCs upon ageing and a bias towards the secretory lineage (Fig 4B). This finding is consistent with reduced Notch1 expression in ISCs (Nalapareddy et al, 2017), which drives differentiation towards the secretory lineage (Pellegrinet et al, 2011; VanDussen et al, 2012). However, it still remains to be elucidated if these changes in ISC cell fate determination and epithelial homeostasis are based solely on ISC‐intrinsic changes or if the surrounding aged stem cell niche is likewise involved.
To identify the molecular mechanism that underlies age‐dependent deterioration of the intestinal epithelium, transcriptomic analyses of fluorescent‐activated cell sorting (FACS)‐sorted ISCs, Paneth and progenitor cells were performed during the last few years (Moorefield et al, 2017; Nalapareddy et al, 2017; Kazakevych et al, 2019; Pentinmikko et al, 2019). These transcriptomic data revealed that canonical Wnt signalling, in particular Wnt3, is reduced in ISCs, Paneth cells and the underlying mesenchyme upon ageing (Fig 4B). Accordingly, WNT3A treatment of organoids from aged mice restored the ageing phenotype of reduced organoid formation efficiency (Nalapareddy et al, 2017). Mechanistically, aged Paneth cells produce increased levels of Notum, a Wnt antagonist, which inhibits canonical Wnt signalling in ISCs (Fig 4B) (Pentinmikko et al, 2019). The authors further identified that deregulation of Notum expression is driven by an altered mTORC1‐PPARa axis in aged Paneth cells. Genetic targeting of Notum or exogenous Wnt activation likewise restored ISC function and organoid formation, while genetic activation of mTORC1 specifically in the intestinal epithelium mimicked an ageing phenotype (Pentinmikko et al, 2019). The reduction in canonical Wnt signalling during ageing might be further enhanced by epigenetic changes since Lgr5 becomes epigenetically silenced by trimethylation on histone H3K27 (Fig 4B) in intestinal organoids from aged mice (Uchida et al, 2018).
Interestingly, it was also shown that Lgr5 expression and ISC function upon ageing could be restored by treatment of organoids with nicotinamide mononucleotide (NMN), a nicotinamide adenine dinucleotide (NAD+) intermediate (Uchida et al, 2018). Accordingly, another recent study showed that NAD+ supplementation and activation of the protein deacetylase Sirt1 improved the functionality of aged ISCs and ameliorated ageing phenotypes (Fig 4B) (Igarashi et al, 2019). These findings suggest a central role of metabolism during ageing‐associated changes in the intestine and highlight the importance of analysing this connection in the future in more detail.
Intestinal metabolism and ageing
Metabolism has been shown to have essential effects on ISC function and intestinal epithelial homeostasis, which are likewise altered by ageing. Thus, the interaction of metabolism and ageing appears to present an intriguing crosstalk and might provide intervention points to prevent age‐associated deterioration of the intestinal epithelium, as observed in elderly individuals (Soenen et al, 2016).
Fasting has been shown to improve ISC function and capacity to form organoids via activation of PPARd/FAO signalling in young mice (Mihaylova et al, 2018). In aged mice, both fasting and direct activation of the PPARd/FAO axis by a small molecule agonist improved ISC function as measured by the capacity of organoid formation (Fig 4C). However, short‐term fasting of 24 h was less effective in improving ISC function in aged mice. This indicates that the positive effect of fasting on ISCs also occurs in aged animals and ameliorates age‐associated deterioration, but it is less dynamic than that in young animals (Mihaylova et al, 2018).
Moreover, in vivo fate‐mapping experiments in aged fasted and control mice revealed an increased number of labelled progenitor cells after short‐term fasting (Mihaylova et al, 2018), hinting towards an improved epithelial turnover rate upon reduced caloric uptake (Fig 4C). This observation indicates that fasting has a positive effect on ISC capacity and epithelial homeostasis not only in young mice but also in aged animals and is able to ameliorate the ageing phenotypes in ISCs.
Another mechanism of how fasting, and thereby dampened mTORC1 activity (Yilmaz et al, 2012), improves ISC function was recently reported (He et al, 2020). The authors identified that increased mTORC1 signalling in aged ISCs and transit amplifying cells induces the MKK6‐p38‐p53 stress response pathway activity. Enhanced p53 expression caused ISC exhaustion and reduced villi functionality, which is accompanied by reduced nutrient absorption upon ageing (Fig 4C). The ageing phenotypes were partially reversed by rapamycin treatment or p38 inhibition, opening a door for therapeutic intervention beyond CR.
At the molecular level, the NAD‐dependent protein deacetylase Sirt1 has been shown to mediate the positive effect of NAD+ supplementation in aged ISCs towards rejuvenation (Fig 4C) (Igarashi et al, 2019). Interestingly, compared to control mice, Sirt1 knockout (KO) mice display elevated numbers of Paneth cells from mid‐age (5–8 months) on (Wellman et al, 2017), which mimics the differentiation bias towards the secretory lineage observed upon ageing (Nalapareddy et al, 2017; Mihaylova et al, 2018; Pentinmikko et al, 2019). Moreover, upon loss of Sirt1, other ageing phenotypes in aged mutant mice compared to those of aged control mice such as the NRF2‐mediated oxidative stress response (Fig 4C) are enhanced (Wellman et al, 2017). These observations suggest that loss of Sirt1 mimics ageing‐related phenotypes in young organisms and, during ageing, enhances them. A similar effect of a stronger transcriptional response upon intervention in an aged versus young organism was seen in the context of high‐fat diet. Here, the transcriptional response, in terms of numbers of differentially regulated genes, upon high‐fat diet was stronger in aged mice than in young mice (Steegenga et al, 2012). These findings suggest that the regulatory network of ISCs becomes less robust and more susceptible to external influences with age, correlating with an overall decline in animal fitness upon ageing. This is in the context of ageing an interesting aspect as changes on the organismal level are intriguing targets for ageing interventions. Thus, it will be important to assess whether this effect is intestine specific or a general organismal mechanism.
Comparative insights
Knowledge on intestinal homeostasis, ageing, metabolism and the interplay of the latter from Drosophila and mouse gives valuable insights in overall invertebrate and vertebrate biology. The two systems share some overlap in regulatory control and physiological responses while also displaying differences in how they respond to metabolic changes and which ageing phenotypes they develop in the intestine.
One central experimental difference between both model systems is currently how ageing phenotypes are assessed. While in Drosophila lifespan curves are indicative for an ameliorated ageing phenotype, such a readout is not feasible in a similar throughput in the mouse system. Here, in contrast, stem cell capacity assays are used to indirectly assess if the ageing phenotype, such as reduced stem cell function, is improved upon a respective intervention. This difference is important to consider when comparing studies from both model organisms, but offers likewise a valuable opportunity to address molecular mechanisms in depth by cross‐species investigations. This is further supported by the great potential of genetic engineering in Drosophila, which can be achieved in a distinctly shorter time frame and on a broader spectrum (Port et al, 2020) compared to mice, providing options for complement studies.
Intestinal homeostasis is strongly linked and influenced by metabolic changes in Drosophila and mouse. Here, it becomes apparent that ISCs in both systems are partially dependent on other intestinal cell types to sense and respond to changes in nutrient availability. In Drosophila, stem cells have an instructive crosstalk with EECs for dynamic adaptation to metabolic changes (Amcheslavsky et al, 2014), while in mice Paneth cells are involved in this process (Yilmaz et al, 2012). Besides such extrinsic metabolic regulations by dietary alterations, intestinal stem cells are also controlled by intrinsic metabolic states. In Drosophila and mice, deletion of the mitochondrial pyruvate carrier Mpc1, which blocks mitochondrial OXPHOS, triggers a proliferative response and an increase of the stem cell pool (Schell et al, 2017). Similar effects on stem cell proliferation are observed in Drosophila and mice upon CR. In Drosophila, this is due to a switch from oxidative to glycolytic metabolism (Mattila et al, 2018).
Intestinal homeostasis is controlled by the number and proliferation rate of stem cells, not only in response to metabolic changes, but also during ageing. In both systems, mTOR signalling plays a central role during intestinal ageing. In Drosophila, manipulation of TORC1 activity directly in the ISCs delays ageing‐related intestinal dysfunction and improves lifespan (Xi et al, 2019). In comparison, in aged mice, elevated mTORC1 activity in Paneth cells partially accounts for reduced ISC capacity by dampening of canonical Wnt signalling activity in the stem cells. This is caused by an mTORC1‐mediated enhanced secretion of the Wnt antagonist NOTUM (Pentinmikko et al, 2019), and in addition, Paneth cells express less Wnt3 as a niche signal for ISCs (Nalapareddy et al, 2017).
From the above, one can conclude that mouse ISCs are in the context of metabolic as well as ageing‐related changes greatly influenced by Paneth cells and form a strong dependent relationship. While the Drosophila intestine is devoid of Paneth cells, stem cells display crosstalks with all the different cell types in distinct contexts. If mouse ISCs are likewise influenced by other intestinal cell types beside Paneth cells during metabolic changes and ageing, remains to be elucidated.
Metabolic changes influence the intestinal epithelium over the entire lifespan of Drosophila and mice, and interplay with cellular alterations upon ageing. In Drosophila and mice, supplementation of NAD+ prevents stem cell ageing, mediated via a Sirt1‐dependent mechanism and probably relying on the regulation of mitophagy (Fang et al, 2019; Igarashi et al, 2019). In terms of dietary changes, fasting causes a metabolic switch in both systems with positive effects on lifespan and ageing phenotypes. In Drosophila, this intervention increases the usage of triglycerides, leading to lifespan extension. In mice, fasting triggers fatty acid oxidation, which similarly improves the function of aged ISCs, indicated by increased organoid formation efficiency. While in Drosophila it is well established that a reduction in Methionine levels in the intestine are associated with improved lifespan (Parkhitko et al, 2016), in mice it is so far only associated with improved barrier function in young high‐fat‐fed mice (Yang et al, 2019). However, since reduced intestinal barrier function is an age‐related phenotype, future studies might shine light on a potential correlation of methionine reduction and improved ISC function during ageing.
Intestinal barrier function in disease and ageing
The maintenance of intestinal barrier function, including its metabolic and immune functions, is essential for organismal health. Intestinal barrier failure has been described as a pathological hallmark of ageing (Chelakkot et al, 2018). Dysfunction of the intestinal barrier is associated with local and systemic disorders, including inflammatory bowel disease, obesity, metabolic diseases, liver disease, and lung and brain dysfunctions (Nicoletti, 2015; Chelakkot et al, 2018; Thaiss et al, 2018). In addition, the age‐related intestinal dysbiosis is associated with intestinal barrier dysfunction in both Drosophila and mice (Clark et al, 2015; Thevaranjan et al, 2017). However, the mechanisms of age‐related increases in intestinal barrier dysfunction and how the intestinal barrier maintains tissue homeostasis upon injury remain unclear.
The intestinal epithelial barrier in Drosophila
The intestinal epithelium forms a selectively permeable barrier that consists of mucus and epithelial cells to allow nutrient absorption and prevent infection by pathogens (Nicoletti, 2015). The Drosophila intestine also contains the peritrophic matrix (PM), a chitinous layer that protects the tissue from physical damage (Fig 5A) (Buchon et al, 2013). Intestinal barrier failure leads to immune activation and systemic metabolic defects, including impaired insulin signalling activity, which may link epithelial barrier defects to ageing (Rera et al, 2012). Furthermore, Clark et al (2015) reported that alterations in microbial composition occur prior to age‐related intestinal barrier defects, resulting in immune activation and impaired excretory function. This age‐related intestinal barrier defect eventually causes systemic immune activation and animal death (Fig 5B) (Clark et al, 2015).
Figure 5. Ageing‐associated intestinal barrier failure.
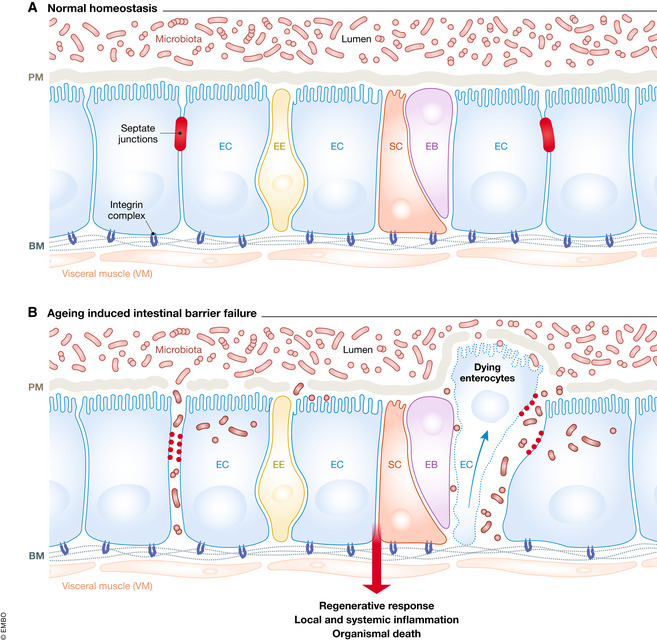
(A) Under normal homeostasis, the intact and healthy intestinal barrier maintains epithelial function and prevents bacterial translocation. (B) Ageing causes septate junctions loss, intestinal barrier dysfunction, microbial dysbiosis, systemic infection and animal death.
To understand the mechanical basis for maintaining the epithelial barrier, several studies have specifically focused on the role of septate junction proteins. For instance, study has demonstrated that barrier integrity relies on the correct positioning of septate junctions in invertebrates and tight junctions in vertebrates (Chen et al, 2018). Alterations in the levels or localization of junction‐associated proteins can lead to impaired intestinal barrier function, ageing‐related ISC proliferation and misdifferentiation (Izumi et al, 2012; Yanagihashi et al, 2012; Byri et al, 2015; Resnik‐Docampo et al, 2017, 2018). For example, Bonnay et al (2013) demonstrated that the septate junction protein big bang (bbg) is required to maintain barrier function. Loss of the septate junction protein Snakeskin (Ssk) leads to rapid intestinal barrier failure, gut morphology changes, microbial dysbiosis and reduced lifespan (Salazar et al, 2018).
Recent studies have also revealed an important role of the basal lamina (BM) in maintaining intestinal barrier function (Kiss et al, 2016). The BM is a layer of extracellular matrix secreted by intestinal epithelial cells. Loss of one key component of BM, type IV collagen (col4a1), leads to a shortened gut, impaired intestinal barrier, immune‐related gene activation and reduced lifespan (Kiss et al, 2016). Drosophila transglutaminase (TG) crosslinks the PM protein Drosocrystallin (Dcy) to form the gut mucosal barrier (Kuraishi et al, 2011; Shibata et al, 2015). Intestinal‐specific loss of Dcy or TG induces cell death, disrupts the epithelial barrier and causes death of flies upon bacterial challenge (Shibata et al, 2015). Consistently, loss of the O‐glycosyltransferase PGANT4 in the larval gut impairs the secretion of PM components, thereby leading to intestinal barrier dysfunction (Zhang et al, 2017). Studies have also shown that JNK activity is required for stimuli‐induced intestinal barrier dysfunction followed by intestinal inflammation and microbial dysbiosis. Reducing JNK activity restores intestinal barrier function and host–microbe homeostasis and extends animal lifespan (Zhou et al, 2017; Zhou & Boutros, 2020). Hence, these data support an important role of epithelial barrier function in the maintenance of intestinal homeostasis to ensure proper nutrient uptake and organismal health during age and challenge conditions.
Intestinal barrier function in mice
Intestinal epithelial cells in mice are connected by tight junctions, including zonula occludens (ZOs), occludins, claudins and junctional adhesion molecules (JAMs) (Tran & Greenwood‐Van Meerveld, 2013). These junction proteins form a continuous and complex architecture with the membrane cytoskeleton to maintain intestinal barrier integrity (Günzel & Yu, 2013; Chelakkot et al, 2018). The tight junction complexes maintain barrier integrity by interactively cooperating with cytoplasmic peripheral membrane proteins and cytoskeleton components such as F‐actin and myosin (Günzel & Yu, 2013). The disruption of tight junctions leads to increased intestinal permeability and the development of intestinal inflammatory diseases (Zeissig et al, 2007; Mir et al, 2016; Almousa et al, 2018; Chelakkot et al, 2018). For example, mice lacking junctional adhesion molecule A (JAM‐A) showed increased intestinal permeability with enhanced proinflammatory signatures, such as accumulation of CD4+ T cells and upregulation of inflammatory cytokines (Khounlotham et al, 2012). Additionally, mutations in genes related to tight junction complexes cause gut barrier dysfunction and often lead to distant organ failure and critical diseases (Cunningham & Turner, 2012; Su et al, 2013; Jin & Blikslager, 2016; Yoseph et al, 2016; Lorentz et al, 2017). In contrast, the expression of occludin prevents stimuli‐induced defects in intestinal permeability and improves intestinal function (McCarthy et al, 1996; Marchiando et al, 2010). These results indicate that reinforcing tight junction function is essential for improving intestinal physiology and may provide a target for the treatment of inflammation, systemic infection and healthspan extension. An age‐related increase in intestinal barrier dysfunction has been described in both animal and human studies (Parrish, 2017). Further investigations have revealed that advanced age promotes not only intestinal barrier failure but also inflammation, microbial translocation and systemic infection in various model organisms, including Drosophila, mice and monkeys (Tran & Greenwood‐Van Meerveld, 2013; Clark et al, 2015; Mitchell et al, 2017; Wen et al, 2019). It remains to be assessed whether developmental signalling pathways, including Wnt pathways, are involved in maintaining the intestinal barrier, similar to its role during skin homeostasis (Augustin et al, 2013).
Outlook
Maintaining intestinal homeostasis is essential as its deterioration followed by epithelial barrier failure has severe detrimental consequences for the whole organism. Ageing and associated metabolic changes influence the physiological responses of the whole organism, ranging from the behaviour of stem cells to changes in plasticity and environmental responses, thereby impacting on intestinal homeostasis. An important frontier and focus of recent research is the communication between organs to get a better understanding how gut homeostasis is linked to overall organismal health. Moreover, it will be important to understand whether and how well the insight from model organisms can be translated to human intestinal homeostasis and ageing. Metabolic and signalling pathways implicated in intestinal homeostasis are often highly homologous in vertebrates and invertebrates and the high frequency of gene homology between Drosophila, mouse and human (Tables 1 and 2) provide a reliable base to identify conserved processes. Studies that assess both invertebrate and vertebrate systems (Perea et al, 2017; Schell et al, 2017; Johansson et al, 2019) are also instrumental in the identification of genes that act in conserved processes during intestinal homeostasis.
The Drosophila intestine and its interaction with other organs has been used as a model in recent years to analyse principal mechanisms of inter‐organ communication (Liu & Jin, 2017). For example, it was found that pro‐hormones secreted by EEs are associated with lipid storage and systemic lipid metabolism in the fat body, the functional homolog of the mammalian liver. Loss of tachykinins, which are secreted by EEs in the intestine, results in elevated lipid levels in the fat body, while an increase in tachykinin levels causes a decrease in systemic lipid storage (Amcheslavsky et al, 2014). The gut–brain axis is another intriguing example of inter‐organ communication. Here, important roles have been attributed to insulin‐like peptides (Dilps), which are mainly derived from insulin‐producing cells in the brain. Dilps have also been found to be involved in the damage‐induced self‐renewal of ISCs (Amcheslavsky et al, 2009) and are generally associated with organismal health and lifespan, paralleling the positive effect of insulin on longevity (Tain et al, 2017). A recent study underlined the role of the gut–brain axis in mammals, showing its involvement in lowering hepatic glucose production upon high‐fat diet in rats via inhibition of mTOR signalling in the upper small intestine (Waise et al, 2019). Further studies of organ‐to‐organ communication will be required to unravel how metabolism and ageing as physiological parameters for organismal health influence intestinal homeostasis and homeostatic regulation in the whole organism.
Recent advances in ex vivo culturing of 3‐dimensional organoids (Clevers, 2016) and combining different human tissues as organs‐on‐a‐chip (Takebe et al, 2017) might open the door for physiologically relevant testing of drugs to influence the metabolism and ageing‐dependent inter‐organ communication.
Another focus of intensive research is the intestinal microbiome. The intestine of animals harbours a complex microbial community that is involved in host food digestion, metabolic adaptation and immune system modulations (Leulier et al, 2017). Increasing evidence suggests an important role of host–microbe interaction in ageing using various model systems, such as nematode, fly and mouse (Cabreiro et al, 2013; Gusarov et al, 2013; Clark et al, 2015; Nguyen et al, 2015). These studies proposed that alterations in microbiota (dysbiosis) could represent an important regulator of ageing‐related processes. In addition, recent studies already began to explore effective ways to improve animal longevity through targeted microbiome alterations (Bana & Cabreiro, 2019; Pryor et al, 2019).
We particularly highlight the role of the intestinal epithelial barrier as an integrator of various internal and external signalling during ageing. Strengthening of this barrier function can be an effective strategy to prevent intestinal inflammation and delay ageing‐related intestinal pathology. Further molecular studies, combining the strengths of genetic model systems and ex vivo organoid cultures, can lay the basis for the development of agents that sustain and improve intestinal barrier function and mitigate its decay in disease and ageing.
Conflict of interest
The authors declare that they have no conflict of interest.
Glossary
- (m)TORC1
(mechanistic) target of rapamycin complex 1
- AL
ad libitum
- AMPK
AMP‐activated protein kinase
- BM
basal lamina
- BMP
bone morphogenetic protein
- CR
caloric restriction
- CREB
cyclic AMP‐responsive element‐binding protein
- Dilps
insulin‐like peptides
- EB
enteroblast
- EC
enterocyte
- EEC
enteroendocrine cell
- EGF
epidermal growth factor
- ER
endoplasmatic reticulum
- FAO
fatty acid oxidation
- FKH
homologue fork head
- GC
goblet cell
- HBP
hexosamine biosynthesis pathway
- HFD
high‐fat diet
- InR
insulin receptor
- ISC
intestinal stem cell
- JAK/STAT
Janus kinase/signal transducers and activators of transcription
- JAM
junctional adhesion molecules
- JNK
c‐Jun N‐terminal kinase
- MAPK
mitogen‐activated protein kinase
- NAD
nicotinamide adenine dinucleotide
- NMN
nicotinamide mononucleotide
- OXPHOS
oxidative phosphorylation
- PC
Paneth cell
- PM
peritrophic matrix
- PPAR
peroxisome proliferator‐activated receptor
- ROS
reactive oxygen species
- SAH
S‐adenosyl‐homocysteine
- SAM
S‐adenosyl‐methionine
- SC
stem cell
- TA
transit amplifying
- TGFβ
transforming growth factor beta
- TG
transglutaminase
- Tk
tachykinin
- TOR
target of rapamycin
- VM
visceral muscle
- Wnt
wingless‐related MMTV integration site 1
- ZO
zona occludens
In need of answers.
During metabolic changes, the EECs in Drosophila and Paneth cells in mice convert instruction to the ISCs for cellular responses. How do intestinal stromal cells, immune cells and microbes impact on the metabolic state of stem cells to influence their behaviour?
In the aged murine intestine, Paneth cells impact on ISCs and account for their reduced capacity; however, it remains to be unravelled what causes the age‐related changes in the Paneth cells. Moreover, it is so far unknown what impact the aged underlying mesenchyme, immune cells and enteric nervous system have on the regeneration potential of the aged intestinal epithelium?
In ageing conditions, dynamic metabolic changes must occur in various intestinal cell types, especially stem cells. Taking advantage of single‐cell technologies, functional omics can be applied to these cells not only for investigating the underlying mechanisms in the ageing process but also uncovering better strategies to prevent ageing effects.
How do ageing‐related changes in the microenvironment or stem cell niche influence intestinal disorders such as inflammatory bowel diseases or cancer? Also, how is the cross communication between the microenvironment, stem cell niche and stromal cells contributing to disease progression?
Supporting information
Appendix
Acknowledgements
We thank Kim Boonekamp and Siamak Redhai for critical comments on the manuscript and members of the Boutros laboratory for helpful discussions. Work in the laboratory of M.B. has been supported in part by DFG SFB873, ERC SYNERGY grant DECODE and the Helmholtz Future Topic AMPro.
EMBO Reports (2020) 21: e50047
See the Glossary for abbreviations used in this article.
References
- Aitman TJ, Boone C, Churchill GA, Hengartner MO, Mackay TFC, Stemple DL (2011) The future of model organisms in human disease research. Nat Rev Genet 12: 575–582 [DOI] [PubMed] [Google Scholar]
- Akagi K, Wilson KA, Katewa SD, Ortega M, Simons J, Hilsabeck TA, Kapuria S, Sharma A, Jasper H, Kapahi P (2018) Dietary restriction improves intestinal cellular fitness to enhance gut barrier function and lifespan in D. melanogaster . PLoS Genet 14: e1007777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almousa AA, Meurens F, Krol ES, Alcorn J (2018) Linoorbitides and enterolactone mitigate inflammation‐induced oxidative stress and loss of intestinal epithelial barrier integrity. Int Immunopharmacol 64: 42–51 [DOI] [PubMed] [Google Scholar]
- Amcheslavsky A, Jiang J, Ip YT (2009) Tissue damage‐induced intestinal stem cell division in Drosophila . Cell Stem Cell 4: 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A, Song W, Li Q, Nie Y, Bragatto I, Ferrandon D, Perrimon N, Ip YT (2014) Enteroendocrine cells support intestinal stem‐cell‐mediated homeostasis in Drosophila. Cell Rep 9: 32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Gross J, Baumann D, Korn C, Kerr G, Grigoryan T, Mauch C, Birchmeier W, Boutros M (2013) Loss of epidermal Evi/Wls results in a phenotype resembling psoriasiform dermatitis. J Exp Med 210: 1761–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyaz A, Kumar S, Sangiorgi B, Ghoshal B, Gosio J, Ouladan S, Fink M, Barutcu S, Trcka D, Shen J et al (2019) Single‐cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569: 121–125 [DOI] [PubMed] [Google Scholar]
- Bana B, Cabreiro F (2019) The microbiome and aging. Annu Rev Genet 53: 239–261 [DOI] [PubMed] [Google Scholar]
- Bárcena C, Quirós PM, Durand S, Mayoral P, Rodríguez F, Caravia XM, Mariño G, Garabaya C, Fernández‐García MT, Kroemer G et al (2018) Methionine restriction extends lifespan in progeroid mice and alters lipid and bile acid metabolism. Cell Rep 24: 2392–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ et al (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007 [DOI] [PubMed] [Google Scholar]
- Beumer J, Clevers H (2016) Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 143: 3639–3649 [DOI] [PubMed] [Google Scholar]
- Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong S‐J, Bauer‐Rowe KE, Xifaras ME, Akkad A, Arias E et al (2016) High‐fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Hochmuth CE, Jasper H (2008) JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3: 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H (2010) Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet 6: e1001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B, Jasper H (2011) EGF signaling regulates the proliferation of intestinal stem cells in Drosophila . Development 138: 1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H (1999) Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116: 7–14 [DOI] [PubMed] [Google Scholar]
- Bolukbasi E, Khericha M, Regan JC, Ivanov DK, Adcott J, Dyson MC, Nespital T, Thornton JM, Alic N, Partridge L (2017) Intestinal fork head regulates nutrient absorption and promotes longevity. Cell Rep 21: 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnay F, Cohen‐Berros E, Hoffmann M, Kim SY, Boulianne GL, Hoffmann JA, Matt N, Reichhart J‐M (2013) big bang gene modulates gut immune tolerance in Drosophila. Proc Natl Acad Sci USA 110: 2957–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borch JM, Qi Y, Riley R, Rabkina L, Jasper H (2017) PGAM5 promotes lasting FoxO activation after developmental mitochondrial stress and extends lifespan in Drosophila . eLife 6: e26952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Chakrabarti S, Lemaitre B (2009) Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila . Genes Dev 23: 2333–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Kuraishi T, Lemaitre B (2010) Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol 8: 152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Broderick NA, Lemaitre B (2013) Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol 11: 615–626 [DOI] [PubMed] [Google Scholar]
- Byri S, Misra T, Syed ZA, Bätz T, Shah J, Boril L, Glashauser J, Aegerter‐Wilmsen T, Matzat T, Moussian B et al (2015) The triple‐repeat protein anakonda controls epithelial tricellular junction formation in Drosophila. Dev Cell 33: 535–548 [DOI] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung K‐Y, Vergara‐Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene NDE, Gems D (2013) Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153: 228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterson JH, Khericha M, Dyson MC, Vincent AJ, Callard R, Haveron SM, Rajasingam A, Ahmad M, Partridge L (2018) Short‐term, intermittent fasting induces long‐lasting gut health and TOR‐independent lifespan extension. Curr Biol 28: 1714–1724.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelakkot C, Choi Y, Kim D‐K, Park HT, Ghim J, Kwon Y, Jeon J, Kim M‐S, Jee Y‐K, Gho YS et al (2018) Akkermansia muciniphila‐derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med 50: e450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C‐H, Luhur A, Sokol N (2015) Lin‐28 promotes symmetric stem cell division and drives adaptive growth in the adult Drosophila intestine. Development 142: 3478–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Lin R, Jin S, Chen R, Xue H, Ye H, Huang Z (2018) The sphingosine‐1‐phosphate/sphingosine‐1‐phosphate receptor 2 axis in intestinal epithelial cells regulates intestinal barrier function during intestinal epithelial cells‐CD4+T‐cell interactions. Cell Physiol Biochem 48: 1188–1200 [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141: 537–561 [DOI] [PubMed] [Google Scholar]
- Cheng C‐W, Biton M, Haber AL, Gunduz N, Eng G, Gaynor LT, Tripathi S, Calibasi‐Kocal G, Rickelt S, Butty VL et al (2019) Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell 178: 1115–1131.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chng W‐BA, Sleiman MSB, Schüpfer F, Lemaitre B (2014) Transforming growth factor β/activin signaling functions as a sugar‐sensing feedback loop to regulate digestive enzyme expression. Cell Rep 9: 336–348 [DOI] [PubMed] [Google Scholar]
- Clark RI, Salazar A, Yamada R, Fitz‐Gibbon S, Morselli M, Alcaraz J, Rana A, Rera M, Pellegrini M, Ja WW et al (2015) Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep 12: 1656–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H (2013) The intestinal crypt, a prototype stem cell compartment. Cell 154: 274–284 [DOI] [PubMed] [Google Scholar]
- Clevers H (2016) Modeling development and disease with organoids. Cell 165: 1586–1597 [DOI] [PubMed] [Google Scholar]
- Cordero JB, Stefanatos RK, Scopelliti A, Vidal M, Sansom OJ (2012) Inducible progenitor‐derived Wingless regulates adult midgut regeneration in Drosophila . EMBO J 31: 3901–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Tang D, Garside GB, Zeng T, Wang Y, Tao Z, Zhang L, Tao S (2019) Wnt signaling mediates the aging‐induced differentiation impairment of intestinal stem cells. Stem Cell Rev Rep 15: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KE, Turner JR (2012) Myosin light chain kinase: pulling the strings of epithelial tight junction function: MLCK‐dependent regulation of tight junction function. Ann N Y Acad Sci 1258: 34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W, Barker N, Low TY, Koo B‐K, Li VSW, Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M et al (2011) Lgr5 homologues associate with Wnt receptors and mediate R‐spondin signalling. Nature 476: 293–297 [DOI] [PubMed] [Google Scholar]
- de Navascués J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, Martínez‐Arias A, Simons BD (2012) Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells: Drosophila midgut homeostasis involves ISC neutral competition. EMBO J 31: 2473–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Gerencser AA, Jasper H (2015) Signal integration by Ca2+ regulates intestinal stem‐cell activity. Nature 528: 212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AJ, He X, Blanc E, Bolukbasi E, Feseha Y, Yang M, Piper MDW (2018) Tissue‐specific transcriptome profiling of Drosophila reveals roles for GATA transcription factors in longevity by dietary restriction. NPJ Aging Mech Dis 4: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Dobson AJ, Houtz PL, Gläßer C, Revah J, Korzelius J, Patel PH, Edgar BA, Buchon N (2015) Regional cell‐specific transcriptome mapping reveals regulatory complexity in the adult Drosophila midgut. Cell Rep 12: 346–358 [DOI] [PubMed] [Google Scholar]
- Escobar KA, Cole NH, Mermier CM, VanDusseldorp TA (2019) Autophagy and aging: maintaining the proteome through exercise and caloric restriction. Aging Cell 18: e12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers P, Tain LS, Nespital T, Goncalves J, Froehlich J, Partridge L (2016) Reduced insulin/insulin‐like growth factor signaling decreases translation in Drosophila and mice. Sci Rep 6: 30290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Liang Q, Lian T, Wu Q, Gaur U, Li D, Yang D, Mao X, Jin Z, Li Y et al (2015) Rapamycin preserves gut homeostasis during Drosophila aging. Oncotarget 6: 35274–35283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang EF, Hou Y, Lautrup S, Jensen MB, Yang B, SenGupta T, Caponio D, Khezri R, Demarest TG, Aman Y et al (2019) NAD+ augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nat Commun 10: 5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer D, Thompson MA, Takhaveev V, Dobson AJ, Kotronaki I, Green JWM, Heinemann M, Tullet JMA, Alic N (2017) RNA polymerase III limits longevity downstream of TORC1. Nature 552: 263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T (2015) The metabolic regulation of aging. Nat Med 21: 1416–1423 [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L (2015) Promoting health and longevity through diet: from model organisms to humans. Cell 161: 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis‐Tsakonas S (2005) Notch signals control the fate of immature progenitor cells in the intestine. Nature 435: 964–968 [DOI] [PubMed] [Google Scholar]
- Gao Y, Yan Y, Tripathi S, Pentinmikko N, Amaral A, Päivinen P, Domènech‐Moreno E, Andersson S, Wong IPL, Clevers H et al (2020) LKB1 represses ATOH1 via PDK4 and energy metabolism and regulates intestinal stem cell fate. Gastroenterology 158: 1389–1401.e10 [DOI] [PubMed] [Google Scholar]
- Garschall K, Dellago H, Gáliková M, Schosserer M, Flatt T, Grillari J (2017) Ubiquitous overexpression of the DNA repair factor dPrp19 reduces DNA damage and extends Drosophila life span. NPJ Aging Mech Dis 3: 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehart H, Clevers H (2019) Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol 16: 19–34 [DOI] [PubMed] [Google Scholar]
- Gregorieff A (2005) Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 19: 877–890 [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL (2015) Yap‐dependent reprogramming of Lgr5 + stem cells drives intestinal regeneration and cancer. Nature 526: 715–718 [DOI] [PubMed] [Google Scholar]
- Günzel D, Yu ASL (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93: 525–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Driver I, Ohlstein B (2013) Injury‐induced BMP signaling negatively regulates Drosophila midgut homeostasis. J Cell Biol 201: 945–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Ohlstein B (2015) Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science 350: aab0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Lucchetta E, Rafel N, Ohlstein B (2016) Maintenance of the adult Drosophila intestine: all roads lead to homeostasis. Curr Opin Genet Dev 40: 81–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Nan Z, Miao C, Jin X, Yang W, Wang Z, Tu Y, Bao H, Lyu J, Zheng H et al (2019) The autophagy‐related gene Atg101 in Drosophila regulates both neuron and midgut homeostasis. J Biol Chem 294: 5666–5676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusarov I, Gautier L, Smolentseva O, Shamovsky I, Eremina S, Mironov A, Nudler E (2013) Bacterial nitric oxide extends the lifespan of C. elegans . Cell 152: 818–830 [DOI] [PubMed] [Google Scholar]
- Hansen M, Rubinsztein DC, Walker DW (2018) Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol 19: 579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramis A‐PG, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJA, Clevers H (2004) De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303: 1684–1686 [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong W‐G, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM et al (2004) BMP signaling inhibits intestinal stem cell self‐renewal through suppression of Wnt‐beta‐catenin signaling. Nat Genet 36: 1117–1121 [DOI] [PubMed] [Google Scholar]
- He D, Wu H, Xiang J, Ruan X, Peng P, Ruan Y, Chen Y‐G, Wang Y, Yu Q, Zhang H et al (2020) Gut stem cell aging is driven by mTORC1 via a p38 MAPK‐p53 pathway. Nat Commun 11: 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C (2000) Mechanisms of changes in basal metabolism during ageing. Eur J Clin Nutr 54: S77–S91 [DOI] [PubMed] [Google Scholar]
- Hong AW, Meng Z, Guan K‐L (2016) The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol 13: 324–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Argmann C, Houten SM, Cantó C, Jeninga EH, Andreux PA, Thomas C, Doenlen R, Schoonjans K, Auwerx J (2011) The metabolic footprint of aging in mice. Sci Rep 1: 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DJ‐K, Jasper H (2019) Control of intestinal cell fate by dynamic mitotic spindle repositioning influences epithelial homeostasis and longevity. Cell Rep 28: 2807–2823.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Guarente L (2016) mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 166: 436–450 [DOI] [PubMed] [Google Scholar]
- Igarashi M, Miura M, Williams E, Jaksch F, Kadowaki T, Yamauchi T, Guarente L (2019) NAD+ supplementation rejuvenates aged gut adult stem cells. Aging Cell 18: e12935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Yanagihashi Y, Furuse M (2012) A novel protein complex, Mesh‐Ssk, is required for septate junction formation in the Drosophila midgut. J Cell Sci 125: 4923–4933 [DOI] [PubMed] [Google Scholar]
- Jeon H‐J, Kim Y‐S, Kim J‐G, Heo K, Pyo J‐H, Yamaguchi M, Park J‐S, Yoo M‐A (2018) Effect of heterochromatin stability on intestinal stem cell aging in Drosophila. Mech Ageing Dev 173: 50–60 [DOI] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA (2009) Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137: 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo M‐J, Blumhagen RZ, Edgar BA (2011) EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila . Cell Stem Cell 8: 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Edgar BA (2012) Intestinal stem cell function in Drosophila and mice. Curr Opin Genet Dev 22: 354–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Ha N, Forés M, Xiang J, Gläßer C, Maldera J, Jiménez G, Edgar BA (2015) EGFR/Ras signaling controls drosophila intestinal stem cell proliferation via capicua‐regulated genes. PLoS Genet 11: e1005634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Blikslager AT (2016) Myosin light chain kinase mediates intestinal barrier dysfunction via occludin endocytosis during anoxia/reoxygenation injury. Am J Physiol Cell Physiol 311: C996–C1004 [DOI] [PubMed] [Google Scholar]
- Johansson J, Naszai M, Hodder MC, Pickering KA, Miller BW, Ridgway RA, Yu Y, Peschard P, Brachmann S, Campbell AD et al (2019) RAL GTPases drive intestinal stem cell function and regeneration through internalization of WNT signalosomes. Cell Stem Cell 24: 592–607.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juricic P, Grönke S, Partridge L (2020) Branched‐chain amino acids have equivalent effects to other essential amino acids on lifespan and ageing‐related traits in Drosophila. J Gerontol A Biol Sci Med Sci 75: 24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabiri Z, Greicius G, Madan B, Biechele S, Zhong Z, Zaribafzadeh H, Edison Aliyev J, Wu Y, Bunte R et al (2014) Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development 141: 2206–2215 [DOI] [PubMed] [Google Scholar]
- Kang M‐J, Vasudevan D, Kang K, Kim K, Park J‐E, Zhang N, Zeng X, Neubert TA, Marr MT, Ryoo HD (2017) 4E‐BP is a target of the GCN2–ATF4 pathway during Drosophila development and aging. J Cell Biol 216: 115–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Kaeberlein M, Hansen M (2017) Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res Rev 39: 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppila TES, Bratic A, Jensen MB, Baggio F, Partridge L, Jasper H, Grönke S, Larsson N‐G (2018) Mutations of mitochondrial DNA are not major contributors to aging of fruit flies. Proc Natl Acad Sci USA 115: E9620–E9629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakevych J, Stoyanova E, Liebert A, Varga‐Weisz P (2019) Transcriptome analysis identifies a robust gene expression program in the mouse intestinal epithelium on aging. Sci Rep 9: 10410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khounlotham M, Kim W, Peatman E, Nava P, Medina‐Contreras O, Addis C, Koch S, Fournier B, Nusrat A, Denning TL et al (2012) Compromised intestinal epithelial barrier induces adaptive immune compensation that protects from colitis. Immunity 37: 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer D, Ali‐Akbarian L (2004) A brief evidence‐based review of two gastrointestinal illnesses: irritable bowel and leaky gut syndromes. Altern Ther Health Med 10: 22–30; quiz 31, 92 [PubMed] [Google Scholar]
- Kiss M, Kiss AA, Radics M, Popovics N, Hermesz E, Csiszár K, Mink M (2016) Drosophila type IV collagen mutation associates with immune system activation and intestinal dysfunction. Matrix Biol 49: 120–131 [DOI] [PubMed] [Google Scholar]
- Koehler CL, Perkins GA, Ellisman MH, Jones DL (2017) Pink1 and Parkin regulate Drosophila intestinal stem cell proliferation during stress and aging. J Cell Biol 216: 2315–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama‐Koganeya A, Kurosawa M, Hirabayashi Y (2015) Differential effects of tissue‐specific deletion of BOSS on feeding behaviors and energy metabolism. PLoS ONE 10: e0133083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama‐Koganeya A, Kurosawa M, Hirabayashi Y (2017) Loss of BOSS causes shortened lifespan with mitochondrial dysfunction in Drosophila. PLoS ONE 12: e0169073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo B‐K, Stange DE, Sato T, Karthaus W, Farin HF, Huch M, van Es JH, Clevers H (2012) Controlled gene expression in primary Lgr5 organoid cultures. Nat Methods 9: 81–83 [DOI] [PubMed] [Google Scholar]
- Kozar S, Morrissey E, Nicholson AM, van der Heijden M, Zecchini HI, Kemp R, Tavaré S, Vermeulen L, Winton DJ (2013) Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell Stem Cell 13: 626–633 [DOI] [PubMed] [Google Scholar]
- Kuraishi T, Binggeli O, Opota O, Buchon N, Lemaitre B (2011) Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster . Proc Natl Acad Sci USA 108: 15966–15971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, Gladyshev VN (2014) Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino acid status. Nat Commun 5: 3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehane MJ (1997) Peritrophic matrix structure and function. Annu Rev Entomol 42: 525–550 [DOI] [PubMed] [Google Scholar]
- Lehner B (2013) Genotype to phenotype: lessons from model organisms for human genetics. Nat Rev Genet 14: 168–178 [DOI] [PubMed] [Google Scholar]
- Leulier F, MacNeil LT, Lee W‐J, Rawls JF, Cani PD, Schwarzer M, Zhao L, Simpson SJ (2017) Integrative physiology: at the crossroads of nutrition, microbiota, animal physiology, and human health. Cell Metab 25: 522–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Nirala NK, Nie Y, Chen H‐J, Ostroff G, Mao J, Wang Q, Xu L, Ip YT (2018) Ingestion of food particles regulates the mechanosensing misshapen‐yorkie pathway in Drosophila intestinal growth. Dev Cell 45: 433–449.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Balachandra S, Ngo S, O'Brien LE (2017) Feedback regulation of steady‐state epithelial turnover and organ size. Nature 548: 588–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R (2008) Paracrine wingless signalling controls self‐renewal of Drosophila intestinal stem cells. Nature 455: 1119–1123 [DOI] [PubMed] [Google Scholar]
- Liu L, Rando TA (2011) Manifestations and mechanisms of stem cell aging. J Cell Biol 193: 257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Jin LH (2017) Organ‐to‐organ communication: a drosophila gastrointestinal tract perspective. Front Cell Dev Biol 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153: 1194–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentz CA, Liang Z, Meng M, Chen C‐W, Yoseph BP, Breed ER, Mittal R, Klingensmith NJ, Farris AB, Burd EM et al (2017) Myosin light chain kinase knockout improves gut barrier function and confers a survival advantage in polymicrobial sepsis. Mol Med 23: 155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Mato JM (2012) S‐adenosylmethionine in liver health, injury, and cancer. Physiol Rev 92: 1515–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchetta EM, Ohlstein B (2017) Amitosis of polyploid cells regenerates functional stem cells in the Drosophila intestine. Cell Stem Cell 20: 609–620.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhur A, Sokol N (2015) Starving for more: nutrient sensing by LIN‐28 in adult intestinal progenitor cells. Fly (Austin) 9: 173–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhur A, Buddika K, Ariyapala IS, Chen S, Sokol NS (2017) Opposing post‐transcriptional control of InR by FMRP and LIN‐28 adjusts stem cell‐based tissue growth. Cell Rep 21: 2671–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis NM, Wang L, Ortega M, Deng H, Katewa SD, Li PW‐L, Karpac J, Jasper H, Kapahi P (2016) Intestinal IRE1 is required for increased triglyceride metabolism and longer lifespan under dietary restriction. Cell Rep 17: 1207–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhao H, Liu F, Zhao H, Kong R, Shi L, Wei M, Li Z (2019) Heparan sulfate negatively regulates intestinal stem cell proliferation in Drosophila adult midgut. Biol Open 8: bio047126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah AT, Van Landeghem L, Gavin HE, Magness ST, Lund PK (2014) Impact of diet‐induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology 155: 3302–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L (2003) Demography of dietary restriction and death in Drosophila. Science 301: 1731–1733 [DOI] [PubMed] [Google Scholar]
- Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, Raleigh DR, Guan Y, Watson AJM, Montrose MH et al (2010) Caveolin‐1‐dependent occludin endocytosis is required for TNF‐induced tight junction regulation in vivo . J Cell Biol 189: 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marianes A, Spradling AC (2013) Physiological and stem cell compartmentalization within the Drosophila midgut. eLife 2: e00886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K, Kirkwood TBL, Potten CS (1998) Age changes in stem cells of murine small intestinal crypts. Exp Cell Res 241: 316–323 [DOI] [PubMed] [Google Scholar]
- Mattila J, Kokki K, Hietakangas V, Boutros M (2018) Stem cell intrinsic hexosamine metabolism regulates intestinal adaptation to nutrient content. Dev Cell 47: 112–121.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE (1996) Occludin is a functional component of the tight junction. J Cell Sci 109(Pt 9): 2287–2298 [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N (2006) Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439: 475–479 [DOI] [PubMed] [Google Scholar]
- Miguel‐Aliaga I, Jasper H, Lemaitre B (2018) Anatomy and physiology of the digestive tract of Drosophila melanogaster . Genetics 210: 357–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, Cheng C‐W, Cao AQ, Tripathi S, Mana MD, Bauer‐Rowe KE, Abu‐Remaileh M, Clavain L, Erdemir A, Lewis CA (2018) Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell 22: 769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano J, McKay J, Dagenais C, Foster‐Brown L, Pognan F, Gadient R, Jacobs RT, Zacco A, Greenberg B, Ciaccio PJ (2004) Modulation of notch processing by γ‐secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci 82: 341–358 [DOI] [PubMed] [Google Scholar]
- Mir H, Meena AS, Chaudhry KK, Shukla PK, Gangwar R, Manda B, Padala MK, Shen L, Turner JR, Dietrich P et al (2016) Occludin deficiency promotes ethanol‐induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice. Biochim Biophys Acta 1860: 765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EL, Davis AT, Brass K, Dendinger M, Barner R, Gharaibeh R, Fodor AA, Kavanagh K (2017) Reduced intestinal motility, mucosal barrier function, and inflammation in aged monkeys. J Nutr Health Aging 21: 354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorefield EC, Andres SF, Blue RE, Van Landeghem L, Mah AT, Santoro MA, Ding S (2017) Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. Aging 9: 1898–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy P, Sándor GO, Juhász G (2018) Autophagy maintains stem cells and intestinal homeostasis in Drosophila. Sci Rep 8: 4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalapareddy K, Nattamai KJ, Kumar RS, Karns R, Wikenheiser‐Brokamp KA, Sampson LL, Mahe MM, Sundaram N, Yacyshyn M‐B, Yacyshyn B et al (2017) Canonical Wnt signaling ameliorates aging of intestinal stem cells. Cell Rep 18: 2608–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Demaria M, Campisi J, Jasper H (2015) Of flies, mice, and men: evolutionarily conserved tissue damage responses and aging. Dev Cell 32: 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TLA, Vieira‐Silva S, Liston A, Raes J (2015) How informative is the mouse for human gut microbiota research? Dis Model Mech 8: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen NN, Rana A, Goldman C, Moore R, Tai J, Hong Y, Shen J, Walker DW, Hur JH (2019) Proteasome β5 subunit overexpression improves proteostasis during aging and extends lifespan in Drosophila melanogaster . Sci Rep 9: 3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti C (2015) Age‐associated changes of the intestinal epithelial barrier: local and systemic implications. Expert Rev Gastroenterol Hepatol 9: 1467–1469 [DOI] [PubMed] [Google Scholar]
- O'Brien LE, Soliman SS, Li X, Bilder D (2011) Altered modes of stem cell division drive adaptive intestinal growth. Cell 147: 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata F, Miura M (2015) Enhancing S‐adenosyl‐methionine catabolism extends Drosophila lifespan. Nat Commun 6: 8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata F, Tsuda‐Sakurai K, Yamazaki T, Nishio R, Nishimura K, Kimura M, Funakoshi M, Miura M (2018) Nutritional control of stem cell division through S‐adenosylmethionine in Drosophila intestine. Dev Cell 44: 741–751.e3 [DOI] [PubMed] [Google Scholar]
- Obniski R, Sieber M, Spradling AC (2018) Dietary lipids modulate Notch signaling and influence adult intestinal development and metabolism in Drosophila. Dev Cell 47: 98–111.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A (2006) The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439: 470–474 [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA (1993) Low methionine ingestion by rats extends life span. J Nutr 123: 269–274 [DOI] [PubMed] [Google Scholar]
- Park J‐H, Chen J, Jang S, Ahn TJ, Kang K, Choi MS, Kwon JY (2016) A subset of enteroendocrine cells is activated by amino acids in the Drosophila midgut. FEBS Lett 590: 493–500 [DOI] [PubMed] [Google Scholar]
- Parkhitko AA, Binari R, Zhang N, Asara JM, Demontis F, Perrimon N (2016) Tissue‐specific down‐regulation of S‐adenosyl‐homocysteine via suppression of dAhcyL1/dAhcyL2 extends health span and life span in Drosophila. Genes Dev 30: 1409–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhitko AA, Jouandin P, Mohr SE, Perrimon N (2019) Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell 18: e13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish AR (2017) The impact of aging on epithelial barriers. Tissue Barriers 5: e1343172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PH, Pénalva C, Kardorff M, Roca M, Pavlović B, Thiel A, Teleman AA, Edgar BA (2019) Damage sensing by a Nox‐Ask1‐MKK3‐p38 signaling pathway mediates regeneration in the adult Drosophila midgut. Nat Commun 10: 4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F (2011) Dll1‐ and Dll4‐mediated Notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140: 1230–1240.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentinmikko N, Iqbal S, Mana M, Andersson S, Cognetta AB, Suciu RM, Roper J, Luopajärvi K, Markelin E, Gopalakrishnan S et al (2019) Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 571: 398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea D, Guiu J, Hudry B, Konstantinidou C, Milona A, Hadjieconomou D, Carroll T, Hoyer N, Natarajan D, Kallijärvi J et al (2017) Ret receptor tyrosine kinase sustains proliferation and tissue maturation in intestinal epithelia. EMBO J 36: 3029–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LW, Artis D (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14: 141–153 [DOI] [PubMed] [Google Scholar]
- Piper MDW, Bartke A (2008) Diet and aging. Cell Metab 8: 99–104 [DOI] [PubMed] [Google Scholar]
- Piper MDW, Soultoukis GA, Blanc E, Mesaros A, Herbert SL, Juricic P, He X, Atanassov I, Salmonowicz H, Yang M et al (2017) Matching dietary amino acid balance to the in silico‐translated exome optimizes growth and reproduction without cost to lifespan. Cell Metab 25: 610–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F, Strein C, Stricker M, Rauscher B, Heigwer F, Zhou J, Beyersdörffer C, Frei J, Hess A, Kern K et al (2020) A large‐scale resource for tissue‐specific CRISPR mutagenesis in Drosophila . eLife 9: e53865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor R, Norvaisas P, Marinos G, Best L, Thingholm LB, Quintaneiro LM, De Haes W, Esser D, Waschina S, Lujan C et al (2019) Host‐microbe‐drug‐nutrient screen identifies bacterial effectors of metformin therapy. Cell 178: 1299–1312.e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Oliveira MP, Khamoui AV, Aparicio R, Rera M, Rossiter HB, Walker DW (2017) Promoting Drp1‐mediated mitochondrial fission in midlife prolongs healthy lifespan of Drosophila melanogaster. Nat Commun 8: 448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redhai S, Pilgrim C, Gaspar P, van Giesen L, Lopes T, Riabinina O, Grenier T, Milona A, Chanana B, Swadling JB et al (2020) An intestinal zinc sensor regulates food intake and developmental growth. Nature 580: 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan JC, Khericha M, Dobson AJ, Bolukbasi E, Rattanavirotkul N, Partridge L (2016) Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. eLife 5: e10956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T, Jones DL, Walker DW (2011) Modulation of longevity and tissue homeostasis by the Drosophila PGC‐1 homolog. Cell Metab 14: 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rera M, Clark RI, Walker DW (2012) Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci USA 109: 21528–21533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik‐Docampo M, Koehler CL, Clark RI, Schinaman JM, Sauer V, Wong DM, Lewis S, D'Alterio C, Walker DW, Jones DL (2017) Tricellular junctions regulate intestinal stem cell behaviour to maintain homeostasis. Nat Cell Biol 19: 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik‐Docampo M, Sauer V, Schinaman JM, Clark RI, Walker DW, Jones DL (2018) Keeping it tight: the relationship between bacterial dysbiosis, septate junctions, and the intestinal barrier in Drosophila. Fly (Austin) 12: 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras‐Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ (2017) Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 543: 424 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Fernandez IA, Qi Y, Jasper H (2019) Loss of a proteostatic checkpoint in intestinal stem cells contributes to age‐related epithelial dysfunction. Nat Commun 10: 1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar AM, Resnik‐Docampo M, Ulgherait M, Clark RI, Shirasu‐Hiza M, Jones DL, Walker DW (2018) Intestinal snakeskin limits microbial dysbiosis during aging and promotes longevity. iScience 9: 229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson LL, Davis AK, Grogg MW, Zheng Y (2016) mTOR disruption causes intestinal epithelial cell defects and intestinal atrophy postinjury in mice. FASEB J 30: 1263–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ et al (2009) Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459: 262 [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell JC, Wisidagama DR, Bensard C, Zhao H, Wei P, Tanner J, Flores A, Mohlman J, Sorensen LK, Earl CS (2017) Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat Cell Biol 19: 1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopelliti A, Bauer C, Yu Y, Zhang T, Kruspig B, Murphy DJ, Vidal M, Maddocks ODK, Cordero JB (2019) A neuronal relay mediates a nutrient responsive gut/fat body axis regulating energy homeostasis in adult Drosophila. Cell Metab 29: 269–284.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Maki K, Hadano J, Fujikawa T, Kitazaki K, Koshiba T, Kawabata S (2015) Crosslinking of a peritrophic matrix protein protects gut epithelia from bacterial exotoxins. PLoS Pathog 11: e1005244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Zeng X, Zhao J, Liu Y, Hou G, Liu H, Hou SX (2016) The lipolysis pathway sustains normal and transformed stem cells in adult Drosophila. Nature 538: 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siudeja K, Nassari S, Gervais L, Skorski P, Lameiras S, Stolfa D, Zande M, Bernard V, Frio TR, Bardin AJ (2015) Frequent somatic mutation in adult intestinal stem cells drives neoplasia and genetic mosaicism during aging. Cell Stem Cell 17: 663–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NR, Gallagher AC, Wong MH (2016) Defining a stem cell hierarchy in the intestine: markers, caveats and controversies. J Physiol 594: 4781–4790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon‐Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD et al (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144 [DOI] [PubMed] [Google Scholar]
- Soenen S, Rayner CK, Jones KL, Horowitz M (2016) The ageing gastrointestinal tract. Curr Opin Clin Nutr Metab Care 19: 12–18 [DOI] [PubMed] [Google Scholar]
- Song W, Veenstra JA, Perrimon N (2014) Control of lipid metabolism by tachykinin in Drosophila. Cell Rep 9: 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Cheng D, Hong S, Sappe B, Hu Y, Wei N, Zhu C, O'Connor MB, Pissios P, Perrimon N (2017) Midgut‐derived activin regulates glucagon‐like action in the fat body and glycemic control. Cell Metab 25: 386–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Owusu‐Ansah E, Hu Y, Cheng D, Ni X, Zirin J, Perrimon N (2017) Activin signaling mediates muscle‐to‐adipose communication in a mitochondria dysfunction‐associated obesity model. Proc Natl Acad Sci USA 114: 8596–8601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa‐Victor P, Ayyaz A, Hayashi R, Qi Y, Madden DT, Lunyak VV, Jasper H (2017) Piwi is required to limit exhaustion of aging somatic stem cells. Cell Rep 20: 2527–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovran B, Hugenholtz F, Elderman M, Beek AAV, Graversen K, Huijskes M, Boekschoten MV, Savelkoul HFJ, Vos PD, Dekker J et al (2019) Age‐associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci Rep 9: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger BZ, Datar R, Murtaugh LC, Melton DA (2005) Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci USA 102: 12443–12448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegenga WT, de Wit NJ, Boekschoten MV, IJssennagger N, Lute C, Keshtkar S, Bromhaar MMG, Kampman E, de Groot LC, Muller M (2012) Structural, functional and molecular analysis of the effects of aging in the small intestine and colon of C57BL/6J mice. BMC Med Genomics 5: 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, Khramtsova EA, Khramtsova G, Tsai P, Fu Y et al (2013) TNFR2 activates MLCK‐dependent tight junction dysregulation to cause apoptosis‐mediated barrier loss and experimental colitis. Gastroenterology 145: 407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Sadighi Akha AA, Miller RA, Harper JM (2009) Life‐span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci 64: 711–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tain LS, Sehlke R, Jain C, Chokkalingam M, Nagaraj N, Essers P, Rassner M, Grönke S, Froelich J, Dieterich C et al (2017) A proteomic atlas of insulin signalling reveals tissue‐specific mechanisms of longevity assurance. Mol Syst Biol 13: 939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe T, Zhang B, Radisic M (2017) Synergistic engineering: organoids meet organs‐on‐a‐chip. Cell Stem Cell 21: 297–300 [DOI] [PubMed] [Google Scholar]
- Takeda K, Okumura T, Terahata M, Yamaguchi M, Taniguchi K, Adachi‐Yamada T (2018) Drosophila peptide hormones allatostatin A and diuretic hormone 31 exhibiting complementary gradient distribution in posterior midgut antagonistically regulate midgut senescence and adult lifespan. Zoolog Sci 35: 75–85 [DOI] [PubMed] [Google Scholar]
- Tatar M, Post S, Yu K (2014) Nutrient control of Drosophila longevity. Trends Endocrinol Metab 25: 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M et al (2018) Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359: 1376–1383 [DOI] [PubMed] [Google Scholar]
- Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP et al (2017) Age‐associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21: 455–466.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Greenwood‐Van Meerveld B (2013) Age‐associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci 68: 1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida R, Saito Y, Nogami K, Kajiyama Y, Suzuki Y, Kawase Y, Nakaoka T, Muramatsu T, Kimura M, Saito H (2018) Epigenetic silencing of Lgr5 induces senescence of intestinal epithelial organoids during the process of aging. NPJ Aging Mech Dis 5: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulgherait M, Rana A, Rera M, Graniel J, Walker DW (2014) AMPK modulates tissue and organismal aging in a non‐cell‐autonomous manner. Cell Rep 8: 1767–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Degirmenci B, Moor AE, Herr P, Zimmerli D, Moor MB, Hausmann G, Cantù C, Aguet M, Basler K (2016) Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep 15: 911–918 [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H (2009) Stem cells, self‐renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–260 [DOI] [PubMed] [Google Scholar]
- VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud A et al (2012) Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 139: 488–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L, Snippert HJ (2014) Stem cell dynamics in homeostasis and cancer of the intestine. Nat Rev Cancer 14: 468–480 [DOI] [PubMed] [Google Scholar]
- Waise TMZ, Rasti M, Duca FA, Zhang S‐Y, Bauer PV, Rhodes CJ, Lam TKT (2019) Inhibition of upper small intestinal mTOR lowers plasma glucose levels by inhibiting glucose production. Nat Commun 10: 714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zeng X, Ryoo HD, Jasper H (2014) Integration of UPRER and oxidative stress signaling in the control of intestinal stem cell proliferation. PLoS Genet 10: e1004568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ryoo HD, Qi Y, Jasper H (2015) PERK limits Drosophila lifespan by promoting intestinal stem cell proliferation in response to ER stress. PLoS Genet 11: e1005220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Rong X, Palladino END, Wang J, Fogelman AM, Martín MG, Alrefai WA, Ford DA, Tontonoz P (2018) Phospholipid remodeling and cholesterol availability regulate intestinal stemness and tumorigenesis. Cell Stem Cell 22: 206–220.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ikuno Y, Kakeya Y, Ikeno S, Taniura H, Kurono M, Minemori K, Katsuyama Y, Naka‐Kaneda H (2019) Age‐related dysfunction of the DNA damage response in intestinal stem cells. Inflamm Regen 39: 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman AS, Metukuri MR, Kazgan N, Xu X, Xu Q, Ren NSX, Czopik A, Shanahan MT, Kang A, Chen W et al (2017) Intestinal epithelial sirtuin 1 regulates intestinal inflammation during aging in mice by altering the intestinal microbiota. Gastroenterology 153: 772–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen SW, Shim R, Ho L, Wanrooy BJ, Srikhanta YN, Prame Kumar K, Nicholls AJ, Sj S, Sepehrizadeh T, Veer M et al (2019) Advanced age promotes colonic dysfunction and gut‐derived lung infection after stroke. Aging Cell 18: e12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisidagama DR, Thummel CS (2019) Regulation of Drosophila intestinal stem cell proliferation by enterocyte mitochondrial pyruvate metabolism. G3 (Bethesda) 9: 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J, Cai J, Cheng Y, Fu Y, Wei W, Zhang Z, Zhuang Z, Hao Y, Lilly MA, Wei Y (2019) The TORC1 inhibitor Nprl2 protects age‐related digestive function in Drosophila . Aging 11: 9811–9828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihashi Y, Usui T, Izumi Y, Yonemura S, Sumida M, Tsukita S, Uemura T, Furuse M (2012) Snakeskin, a membrane protein associated with smooth septate junctions, is required for intestinal barrier function in Drosophila. J Cell Sci 125: 1980–1990 [DOI] [PubMed] [Google Scholar]
- Yang Q (2001) Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294: 2155–2158 [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhang Y, Xu Y, Luo T, Ge Y, Jiang Y, Shi Y, Sun J, Le G (2019) Dietary methionine restriction improves the gut microbiota and reduces intestinal permeability and inflammation in high‐fat‐fed mice. Food Funct 10: 5952–5968 [DOI] [PubMed] [Google Scholar]
- Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer‐Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M et al (2012) mTORC1 in the Paneth cell niche couples intestinal stem‐cell function to calorie intake. Nature 486: 490–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoseph BP, Klingensmith NJ, Liang Z, Breed ER, Burd EM, Mittal R, Dominguez JA, Petrie B, Ford ML, Coopersmith CM (2016) Mechanisms of intestinal barrier dysfunction in sepsis. Shock 46: 52–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi M, Nakauka‐Ddamba A, Berry CT, Li N, Schoenberger J, Simeonov KP, Cedeno RJ, Yu Z, Lengner CJ (2018) Calorie restriction governs intestinal epithelial regeneration through cell‐autonomous regulation of mTORC1 in reserve stem cells. Stem Cell Rep 10: 703–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke J‐D (2007) Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56: 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Boquete J‐P, Lemaitre B (2017) A genetic framework controlling the differentiation of intestinal stem cells during regeneration in Drosophila . PLoS Genet 13: e1006854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Turner B, Ribbeck K, Ten Hagen KG (2017) Loss of the mucosal barrier alters the progenitor cell niche via Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling. J Biol Chem 292: 21231–21242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N (2018) Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim Nutr 4: 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Holowatyj AN, Roy T, Pronovost SM, Marchetti M, Liu H, Ulrich CM, Edgar BA (2019) An SH3PX1‐dependent endocytosis‐autophagy network restrains intestinal stem cell proliferation by counteracting EGFR‐ERK signaling. Dev Cell 49: 574–589.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Florescu S, Boettcher A‐L, Luo L, Dutta D, Kerr G, Cai Y, Edgar BA, Boutros M (2015) Dpp/Gbb signaling is required for normal intestinal regeneration during infection. Dev Biol 399: 189–203 [DOI] [PubMed] [Google Scholar]
- Zhou J, Edgar BA, Boutros M (2017) ATF3 acts as a rheostat to control JNK signalling during intestinal regeneration. Nat Commun 8: 14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Boutros M (2020) JNK‐dependent intestinal barrier failure disrupts host–microbe homeostasis during tumorigenesis. Proc Natl Acad Sci USA 117: 9401–9412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick RK, Ohlstein B, Klein OD (2018) Intestinal renewal across the animal kingdom: comparing stem cell activity in mouse and Drosophila . Am J Physiol Gastrointest Liver Physiol 316: G313–G322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix


