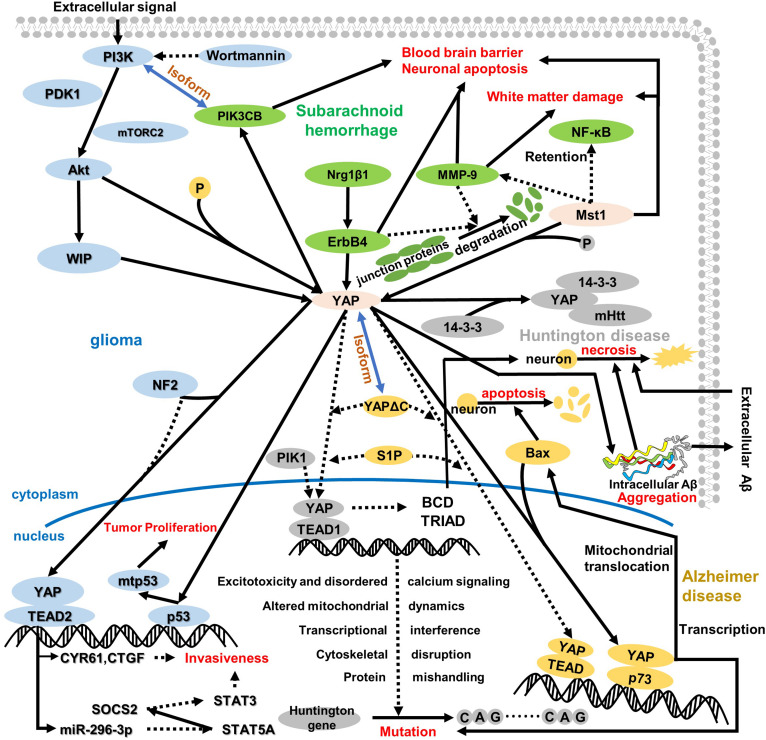
FIGURE 3.
An overview of the role of YAP in the cytoplasm and nucleus regarding central nervous system disease. YAP plays a pivotal role in the whole pathologic process, which depends on its location and pathway. In glioma, YAP is regulated by WIP to induce p53 mutation in the nucleus, further resulting in tumor proliferation and attenuating contact cell inhibition. Of note, WIP expression is regulated via the P13K/Akt pathway, which is assisted by some regulatory proteins, like mTORC2 and PDK1. Meanwhile, NF2 suppresses the interaction between YAP and TEAD2, which triggers transcription target gene CYR61 and CTGF expressions and mediates miR-296-3p regulation. Both CYR61/CTGF and miR-296-3p can inhibit glioma invasiveness, whereas the invasiveness for miR-296-3p is fulfilled by decreased SATA3 and SATA5A and increased SOCS2. After subarachnoid hemorrhage, ErbB4 and Mst1 could respond to the BBB disruption, neuronal apoptosis, and white matter damage. One pathway regarding ErbB4 is upregulation of Nrg1β1, ErbB4, and PIK3CB; another pathway on Mst1 is the degradation of tight junction protein and inhibition of MMP-9 and NF-κB. Intriguingly, the isoform relationship between PI3K and PIK3CB connects glioma and subarachnoid hemorrhage. Additionally, HD is closely related to AD as a neurodegenerative disease. In HD, both decreased interaction between YAP and TEAD1 and increased YAP phosphorylation induce Huntington gene mutation. In the nucleus, the downregulation of YAP/TEAD1 stimulates CAG sequence repetition via excitotoxicity and disordered calcium signaling, altered mitochondrial dynamics, transcriptional interference, cytoskeletal disruption, and protein mishandling. Moreover, the decreased nuclear location of YAP inhibits BCD and TRIAD in cells, which stimulates neuro-necrosis. More interestingly, the mutant Huntington gene interacting with the YAP 14-3-3 complex aggravates the original pathologic symptom. In AD, YAP remarkably reduces interaction with TEAD and augments the combination with p73 that triggers Bax expression to aggravate neuron apoptosis, and YAP/p73 also aggravates the mutation in HD. Moreover, the cytoplasm retention of YAP and TRIAD/BCD mediates intracellular Aβ aggregation further, which results in neuron cell necrosis different from neuron apoptosis. Incidentally, intracellular Aβ aggregation transports outside to accumulate extracellular Aβ aggregation during the late period in AD, and extracellular Aβ aggregation can also result in neuro-necrosis with non-cell-autonomous approaches.