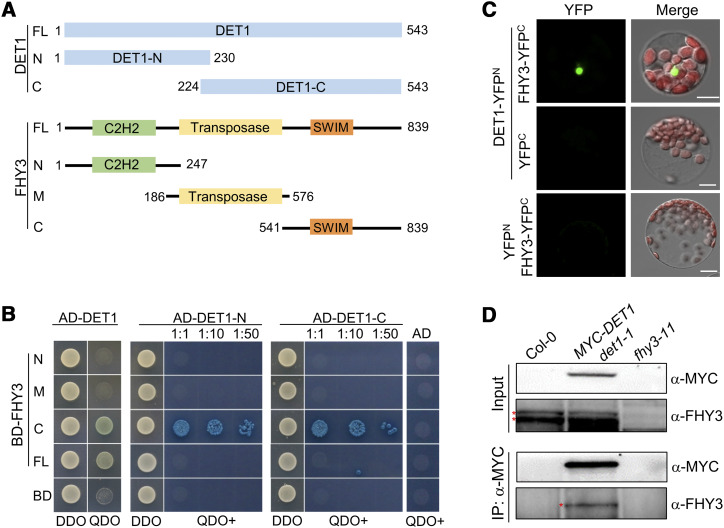
Figure 2.
DET1 physically interacts with FHY3 in vitro and in vivo. A and B, Yeast two-hybrid assays showing DET1 interacts with FHY3 in vitro. The positions of various fragments that used in the yeast two-hybrid assays are shown in A. The various fragments of DET1 and FHY3 were fused to DNA binding domain (BD) or activation domain (AD) of GAL4, respectively. 1:10 and 1:50 indicate the dilutions of yeast cells that were spotted on plates for X-α-gal assays. DDO, Yeast synthetic medium without Trp/Leu; QDO, yeast synthetic medium without Trp/Leu/His/Ade, but with 20 μg mL−1 of X-α-gal and 125 ng mL−1 of AbA; QDO+, QDO medium-plus with 40 μg mL−1 of X-α-gal and 250 ng mL−1 of AbA. C, BiFC assays showing DET1 interacts with FHY3 in Arabidopsis protoplasts. YFPN and YFPC indicate the N- or C-terminal parts of YFP, respectively. Scale bars = 5 μm. D, Co-IP assays showing DET1 interacts with FHY3 in Arabidopsis. Four-day-old various etiolated seedlings were irradiated with light for 30 min and then used to perform Co-IP assays. The fhy3-11 was used as a negative control showing the positions of endogenous FHY3 protein (indicates by asterisk) that detected by anti-FHY3 polyclonal antibodies. Anti-MYC monoclonal antibodies were used to perform the IP and detect the abundance of the MYC-DET1 fusion protein.