Fig. 4. SLC38A2 deficiency causes compartmentalized redox crisis and switch to catabolic metabolism.
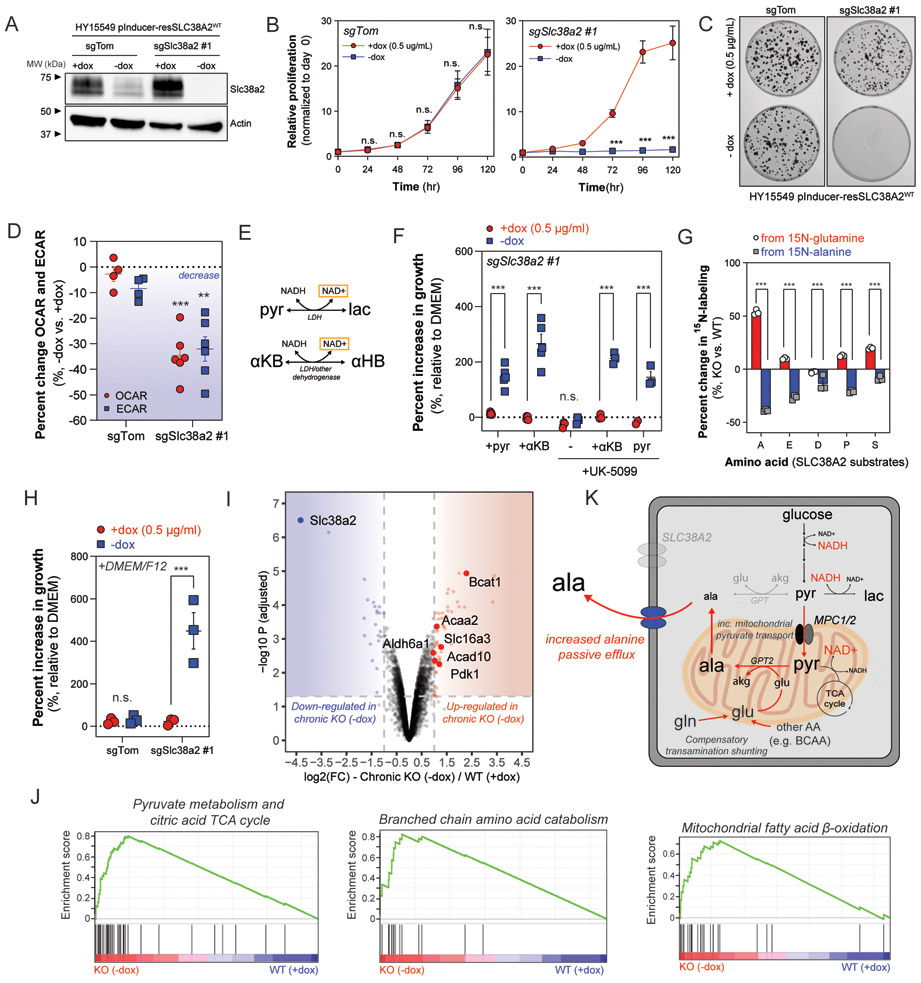
(A) Immunoblot of Slc38a2 and Actin in whole cell lysates (30 μg) collected from clonally derived HY15549 control (sgTom) and Slc38a2-deficient (sgSlc38a2 #1) cells ectopically expressing sgRNA-resistant SLC38A2 cDNA under control of doxycycline-inducible promoter. Cells were cultured in the presence of dox (0.5 μg/ml) or after washout of dox for 24 hours prior to lysis. Withdrawal of doxycycline leads to rapid decrease in SLC38A2 expression in Slc38a2-deficient HY15549 cells. Representative immunoblot of two independent experiments. (B and C) Cell proliferation (B) and representative plates from clonogenic assay (C) of clonally derived control (sgTom) or Slc38a2-deficient (sgSlc38a2 #1) HY15549 cells cultured in DMEM +dox or −dox over five days (B) or 7-10 days (C). Data in B are plotted as cell proliferation relative to day 0 collected after cell attachment. Error bars in B depict s.e.m. of ≥3 independent experiments of four technical replicate wells for each time point. Representative clonogenic plates in C selected from ≥3 independent experiments of three technical replicate plates for each condition. (D) Decrease in extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) in clonally derived HY15549 control (sgTom) or Slc38a2-deficient (sgSlc38a2 #1) cell lines after withdrawal of doxycycline for 24 hours. Data are plotted as a percent increase or decrease of −dox compared to +dox (0.5 μg/ml). Error bars depict s.e.m. of ≥4 independent experiments of ≥10 technical replicate wells for each condition. (E) Pyruvate (pyr) is reduced by NADH by LDH to produce lactate (lac) and regenerate NAD+. α-ketobutyrate (αKB) can be reduced by LDH or other dehydrogenases to produce α-hydroxybutyrate (αHB) and regenerate NAD+. (F) Percent increase in proliferation of HY15549 Slc38a2-deficient cells in DMEM supplemented with doxycycline (+dox 0.5 μg/ml) or without doxycycline (-dox). Cells were supplemented with control (blank DMEM), pyruvate (1mM), α-ketobutyrate (1mM) and either vehicle (DMSO) or UK-5099 (25 μM) and allowed to grow for 96 hours. Data are plotted as a percent increase compared to control DMEM. Error bars depict s.e.m. of ≥3 independent experiments of four technical replicate wells for each condition. (G) Stable isotope labeling from either 15N-alanine or α-15N-glutamine in intracellular alanine (A), glutamate (E), aspartate (D), proline (P), or serine (S) after 24 hours incubation with either tracer. Data are plotted as a percent increase or decrease in HY15549 Slc38a2-deficient cells cultured without doxycycline (KO, -dox) or with doxycycline (WT, +dox 0.5 μg/ml). Error bars depict s.d. of ≥3 technical replicate wells from one independent experiment per tracer. (H) Percent increase in proliferation of HY15549 Slc38a2-deficient cells in DMEM supplemented with doxycycline (+dox 0.5 μg/ml) or without doxycycline (-dox). Cells were supplemented with DMEM or 50:50 DMEM/F12 and allowed to grow for 96 hours. Data are plotted as a percent increase compared to control DMEM. Error bars depict s.e.m. of ≥3 independent experiments of four technical replicate wells for each condition. (I) Volcano plot of differentially expressed proteins between chronic Slc38a2 knockout (chronic KO −dox) and Slc38a2-expressing (WT +dox 0.5 μg/ml) HY15549 cells. Proteins were considered differentially expressed if log2 fold change (FC) ≥ 1 or ≤ −1 and adjusted p-value ≤ 0.05. Metabolic enzymes involved in BCAA catabolism (Bcat1, Aldh6a1), mitochondrial fatty acid β-oxidation (Acaa2, Acad10), and pyruvate metabolism (Slc16a3, Pdk1) that are highly expressed in chronic knockout cells are highlighted with red squares and labeled with protein name. (J) Gene set enrichment analysis (GSEA) of differentially expressed proteins between chronic Slc38a2 knockout (KO −dox) and Slc38a2-expressing (WT +dox). (K) Diagram depicting compartmentalized redox crisis occurring as a result of increased passive efflux of alanine in SLC38A2-deficient cells. Increased mitochondrial pyruvate transport results in compartmentalized NAD(H) crisis reducing both glycolytic and respiratory potential. Decreased utilization of alanine and increased synthesis shifts other amino-nitrogen donors (e.g. glutamine, BCAA) towards mitochondrial de novo alanine synthesis. Significance determined with two-way ANOVA using Sidak’s multiple comparison testing in B, D, F, G, and H. * p<0.05, ** p<0.01, *** p<0.001.
