Abstract
The brain is the most important organ of the human body, and the conversations between the brain and an apparatus can not only reveal a normally functioning or a dysfunctional brain but also can modulate the brain. Here, the apparatus may be a nonbiological instrument, such as a computer, and the consequent brain–computer interface is now a very popular research area with various applications. The apparatus may also be a biological organ or system, such as the gut and muscle, and their efficient conversations with the brain are vital for a healthy life. Are there any common bases that bind these different scenarios? Here, we propose a new comprehensive cross area: Bacomics, which comes from brain–apparatus conversations (BAC) + omics. We take Bacomics to cover at least three situations: (1) The brain is normal, but the conversation channel is disabled, as in amyotrophic lateral sclerosis. The task is to reconstruct or open up new channels to reactivate the brain function. (2) The brain is in disorder, such as in Parkinson’s disease, and the work is to utilize existing or open up new channels to intervene, repair and modulate the brain by medications or stimulation. (3) Both the brain and channels are in order, and the goal is to enhance coordinated development between the brain and apparatus. In this paper, we elaborate the connotation of BAC into three aspects according to the information flow: the issue of output to the outside (BAC-1), the issue of input to the brain (BAC-2) and the issue of unity of brain and apparatus (BAC-3). More importantly, there are no less than five principles that may be taken as the cornerstones of Bacomics, such as feedforward and feedback control, brain plasticity, harmony, the unity of opposites and systems principles. Clearly, Bacomics integrates these seemingly disparate domains, but more importantly, opens a much wider door for the research and development of the brain, and the principles further provide the general framework in which to realize or optimize these various conversations.
Keywords: Bacomics, Brain–apparatus conversation (BAC), Brain disorder, Brain development, Brain reactivation
Introduction
The brain is the most important organ of our human body. Our high-quality life with its independence, mobility and communication is based on the normal functioning of the brain, various organs outside the brain, and the interactions between the brain and the organs connected to it. Unfortunately, hundreds of thousands of people suffer from brain diseases or disorders of the brain’s input/output pathways to peripheral nerves and muscles, for instance, the paralyzing disorders caused by spinal cord injury, brain paralysis, brainstem stroke, amyotrophic lateral sclerosis, depression, autism, schizophrenia, and aphasia (Chaudhary et al. 2016; Hochberg et al. 2006, 2012). These diseases/disorders lead people to lose their normal abilities to communicate and interact with the inside/outside world or engage in the basic social participation needed for a normal life.
To improve the quality of life, the brain–computer interface (BCI) was innovated as a technology to bypass the normal biological output/input channel, and BCI was verified to be an efficient method (Kubler et al. 2006; Mak and Wolpaw 2009; Vidal 1973). The main body of BCI applications is to translate brain signals into commands to control external assistive devices, which could restore mobility and independence for people with motor impairments (Wolpaw et al. 2002). At the initial stage of development, BCI was mainly used to decode the intentions of the users for communication and control in a unidirectional manner (Wolpaw et al. 2000). In recent decades, conceptual categories and technologies have rapidly developed and extended beyond the original framework of BCI. The extensions include a few aspects that are not independent but interrelated: conceptual frameworks, technological innovations, the objects of interaction and the applications.
The emergence of the various conceptual frameworks
In addition to BCI, many terminologies have emerged to define and describe various interface systems, such as brain–machine interface (BMI) (Donoghue 2002; Graf and Andersen 2014; Moxon and Foffani 2015), brain-to-brain interface (BBI) (Grau et al. 2014; Lee et al. 2017; Rao et al. 2014), neurofeedback (Ramot et al. 2017; Sitaram et al. 2017), biological–machine system integration (BMSI) (Lovell et al. 2010), brain–machine–brain interface (BMBI) (O’Doherty et al. 2011), neural interface systems (NIS) (Donoghue 2008; Hatsopoulos and Donoghue 2009), and human brain/cloud interface (B/CI) (Martins et al. 2019). To capture the end-to-end definition and rapid development, any of the listed terminologies may not be appropriate for embracing all systems and applications, and the various terminologies used in different parts of literatures may confuse the scientific community to some extent.
The changing technological innovations
First, the control signals to convey the intention of the users have extended across noninvasive and invasive modalities, and have included the scalp electroencephalography (EEG), magnetoencephalography (MEG), functional MRI (fMRI), functional near-infrared spectroscopy (fNIRS), electrocorticography (ECoG), local field potential (LFP), spikes, electromyography (EMG), and electrooculogram (EOG) signals. Different modalities have particular advantages and disadvantages, and have been applied in different scenarios. The comparisons between these modalities can be found in (Min et al. 2010; Nicolas-Alonso and Gomez-Gil 2012; Ramadan and Vasilakos 2017). Second, new technologies have been developed and include various types of brain stimulation (Gharabaghi et al. 2014). These technologies have brought about changes; in particular, the information transmission modes include not only reading the neural activity from the brain but also inputting digital information back into the brain (Deuschl et al. 2006; Inman et al. 2018; Zrenner et al. 2016). The invasive brain stimulation approaches include vagus nerve stimulation (VNS) (George et al. 2000; Liu et al. 2013) and deep brain stimulation (DBS) (Benabid et al. 2009; Herron et al. 2017), optogenetic stimulation (Iaccarino et al. 2016), etc. Noninvasive approaches include music intervention (He et al. 2017; Hegde 2014), video game interference (Anguera et al. 2013; Franceschini et al. 2013; Nouchi et al. 2012), transcranial magnetic stimulation (TMS) (Hallett 2000; Pascual-Leone et al. 1996; Perera et al. 2016), transcranial direct-current stimulation (tDCS) (Fregni et al. 2005; Kuo et al. 2014; Nitsche et al. 2009), ultrasound stimulation (Wang et al. 2020), functional electrical stimulation (FES) (Biasiucci et al. 2018; Pfurtscheller et al. 2003; Yan et al. 2005), transcutaneous auricular vagus nerve stimulation (taVNS) (Rong et al. 2016), and acupuncture (Eun-Sun et al. 2018; Wang et al. 2012). Each approach may have its particular high-priority application. For example, DBS has been efficient for the treatment of Parkinson’s disease (Benabid et al. 2009), and FES combined with BCI has been valuable for motor recovery after stroke (Biasiucci et al. 2018). Currently, the interactive mode has extended from the open-loop unidirectional emitting or receiving information to closed-loop bidirectional communication (Luu et al. 2016; Shenoy and Carmena 2014; Sitaram et al. 2017). More recently, a concept referred to as a neural coprocessor was proposed to address the simultaneous decoding and encoding for closed-loop control in a unifying framework with two deep recurrent artificial networks (Rao 2019). Third, pharmacological or physical treatment is also a typical interaction channel. When the biological molecule related functions of the brain are problematic, pharmacological treatment may be a choice. For example, amphetamine and methylphenidate have long been used in the treatment of attention deficit hyperactivity disorder (ADHD) in children and adolescents (Berman et al. 2009). Another example is using acupuncture and moxibustion to treat Crohn’s disease (CD), which is a chronic inflammatory bowel disease (IBD), indicating gut–brain axis dysfunction (Bao et al. 2017; Liu et al. 2018). Numerous recent studies have shown that structural and functional abnormalities of the brain may play crucial roles in the development of CD.
Extension in the interaction objects
Previously, the brain is the center and source of control signals, and the modulated object. The main interacting parts are the brain and external devices and the environment, such as the computer and assistive devices (Long et al. 2012; Yu et al. 2012; Zhang et al. 2016a), and the simulated visual environment (Coogan and He 2018; Gateau et al. 2015, 2018; Xiao et al. 2019). Currently, the notion of the interaction objects has been extended such that, in addition to the brain and the nonbiological instrument, these interactions may be between the brain and biological organisms outside of the body and between the brain and the organs inside the body. Regarding the former, the interactions may be between two brains (Grau et al. 2014; Lee et al. 2017; Rao et al. 2014), multiple interconnected brains (Jiang et al. 2019a; Pais-Vieira et al. 2015), and the brain and another biological organism (Li and Zhang 2016; Yoo et al. 2013; Zhang et al. 2019). Regarding the latter, interactions occur between the brain and the muscle system (Ajiboye et al. 2017; Bouton et al. 2016; Do et al. 2011), the peripheral physiological systems (Gorelick et al. 2017), such as the gut–brain axis (Agusti et al. 2018; Bonaz et al. 2018; Foster and McVey Neufeld 2013; Mayer 2011), the hypothalamic–pituitary–adrenal (HPA) axis (Dallman et al. 2003; Sapolsky 2015; Torres-Berrio and Nava-Mesa 2019), and the heart (Catrambone et al. 2019; Faes et al. 2015), etc.
Regarding the interactions between the brain and systemic organs, in recent years, the gut–brain axis has attracted much interest. Some studies have demonstrated that bidirectional communication exists between the brain and the gut microbiota, and the gut microbiota plays a very important role in the development and function of the central nervous system (CNS) through specific channels, such as metabolic, neuroendocrine, and immune pathways (Cerdo et al. 2017; Cryan and Dinan 2012; Diaz Heijtz et al. 2011). The brain–heart interactions are also an interesting topics (Pereira et al. 2013; Samuels 2007; Silvani et al. 2016; Van der Wall and Van Gilst 2013). For instance, a recent study showed that the prefrontal brain regions can modulate vagal control of heart rate at rest (Patron et al. 2019). In fact, brain–heart interactions are bidirectional, the measures of the directed interaction can be achieved by Granger causality (GC) and transfer entropy (TE) (Faes et al. 2015). During sleep, the brain–heart interactions are dynamic across different sleep stages that the information interaction become weaker from light sleep to deep sleep (Faes et al. 2014). Besides, the imbalanced brain–heart interaction could result in a negative impact on health (Silvani et al. 2016). Another example is thyroid hormone, which is essential for normal brain development (Zoeller et al. 2002). The deficit or excess of thyroid hormone during development can have permanent effects on adult neurological function, and maternal levels of thyroid hormone can affect the neurological outcomes of the fetus and neonate. The other systemic organs, e.g., the liver, kidney, lung, endocrine and immune systems, age-related alterations also could negatively influence brain health. In turn, brain dysfunction and damage caused by age and age-related systemic diseases may lead to deleterious effects on the cardiovascular system, which results in alterations of neurohumoral mechanisms and cardiac damage and hypertension. Another distinct example is the interplay between the brain and peripheral physiology under stress (Sapolsky 2015; Torres-Berrio and Nava-Mesa 2019). The stress response involves the activation of the sympathetic nervous system and the HPA axis, and the stress exposure could result in alterations in cognition, emotion and behavior by endocrine transducers of stress.
The change in applications
Regarding the application of these technologies, classical BCIs have been used for typing on computer screens (Krusienski et al. 2008; Salvaris and Sepulveda 2009; Xu et al. 2013), exoskeleton and prostheses control (Lopez-Larraz et al. 2016; Muller-Putz and Pfurtscheller 2008; Schwartz et al. 2006; Wang et al. 2018), 2D cursor control (Li et al. 2010; Wolpaw and McFarland 2004; Wolpaw et al. 1991), assistive technology applications (Cincotti et al. 2008; Rebsamen et al. 2007, 2010), etc. Recently, the applications have extended widely beyond communication and control (Blankertz et al. 2016), and medical applications (Abdulkader et al. 2015; Van Erp et al. 2012). The fields have already broadened to include rehabilitation (Ang et al. 2009; Frisoli et al. 2012; Moldoveanu et al. 2019), neurofeedback (Arns et al. 2009; Ramot et al. 2017; Thibault et al. 2016), neuromodulation (Bashivan et al. 2019; Grossman et al. 2017; Lubianiker et al. 2019; Ponce et al. 2019; Reinhart and Nguyen 2019), treatment of neurological and psychiatric disorders (Daly and Wolpaw 2008; Fan et al. 2018; Lim et al. 2012; McFarland et al. 2017), mental state monitoring and evaluation (Chin-Teng et al. 2010; Dimitrakopoulos et al. 2018), cognitive improvement (Cinel et al. 2019; Lee et al. 2013), gaming and entertainment (Beveridge et al. 2019), neuromarketing (Khushaba et al. 2013; Vecchiato et al. 2011), research tools in cognition and behavior (Arico et al. 2018; Hu et al. 2017; Jensen et al. 2011; Toppi et al. 2016), etc. More applications can be found in (Blankertz et al. 2016; Moxon and Foffani 2015; Rao 2013; Van Erp et al. 2012). In addition, advanced visual prostheses (Lovell et al. 2010), auditory prostheses (Bierer and Middlebrooks 2002) and, mechanical prostheses (Gilja et al. 2011; Velliste et al. 2008) for sensory loss, are currently under development.
Interactions beyond BCI
In addition to the interaction objects mentioned above, interactions between the living environment and the brain has been not properly addressed. Increasing evidence had demonstrated that human brain development and maturation are shaped by environmental exposures for better or worse (Kim et al. 2013; McEwen 2012; McEwen and Gianaros 2010; Meyer-Lindenberg and Tost 2012; Tost et al. 2015). For instance, early maltreatment, social exclusion, and stressful life events can influence brain health. In contrast, highly nurturing maternal care is beneficial for the enhancement of neural plasticity, development of mesolimbic dopaminergic pathways, and enhancement of social and reproductive behaviors. For individuals, an important aspect of the environmental surroundings is their socioeconomic status, which is related to the development of brain function and structure (Chan et al. 2018; Kim et al. 2013; Noble et al. 2012). A study demonstrated that socioeconomic status can shape functional network organization and anatomy of the brain across adult middle age (Chan et al. 2018). It is apparent that these interactions are important for brain health and should be integrated together.
From the reviewed progress and changes, this domain, starting with BCI, has advanced from conventional applications that provide novel channels for communication and control to a comprehensive cross area for engineering, clinical, cognitive and other scientific research areas, and no unified framework has embraced the conceptual depth and developments in this field. In 2010, at the first Chinese brain–computer interface competition workshop (Oct. 26, 2010, Tsinghua University in Beijing), Dr. Dezhong Yao was invited to give a keynote speech, and he chose a title “From Brain–Computer Interface (BCI) to Brain–Apparatus Interaction”. Since then, we began to argue for the necessity of a unified framework for brain–computer and brain–biological organ interactions. Actually, the word “apparatus” appeared in a seminal paper where Vidal argued that “Can these observable electrical brain signals be put to work as carriers of information in man–computer communication or for the purpose of controlling such external apparatus as prosthetic devices or spaceships?” (Vidal 1973), and the word “apparatus” also has appeared in biological communication studies as “there is a noticeable evolutionary direction toward the development of a biological communication apparatus that supported ever more sophisticated forms of speech, or increased communication complexity, culminating in the development of complex speech by Homo sapiens” (Kock 2005).
As the all noted above, BCI and other existed terminologies are not be appropriate for embracing all systems and applications. Accordingly, in this paper, a framework, i.e., Brain–Apparatus Conversation (BAC), is provided to integrate the various concepts, technologies, approaches and applications into a unified area. In the BAC, the apparatus denotes both the nonbiological computer/instruments and biological organs/systems, conversation denotes the unidirectional or bidirectional communication between the brain and apparatus. Finally, BAC is further updated as a new comprehensive cross area, Bacomics (BAC + omics), with some basic principles being summarized for guiding BAC in the future. At the end, we also present the opportunity and challenges in this area.
Brain–apparatus conversation (BAC): concept and framework
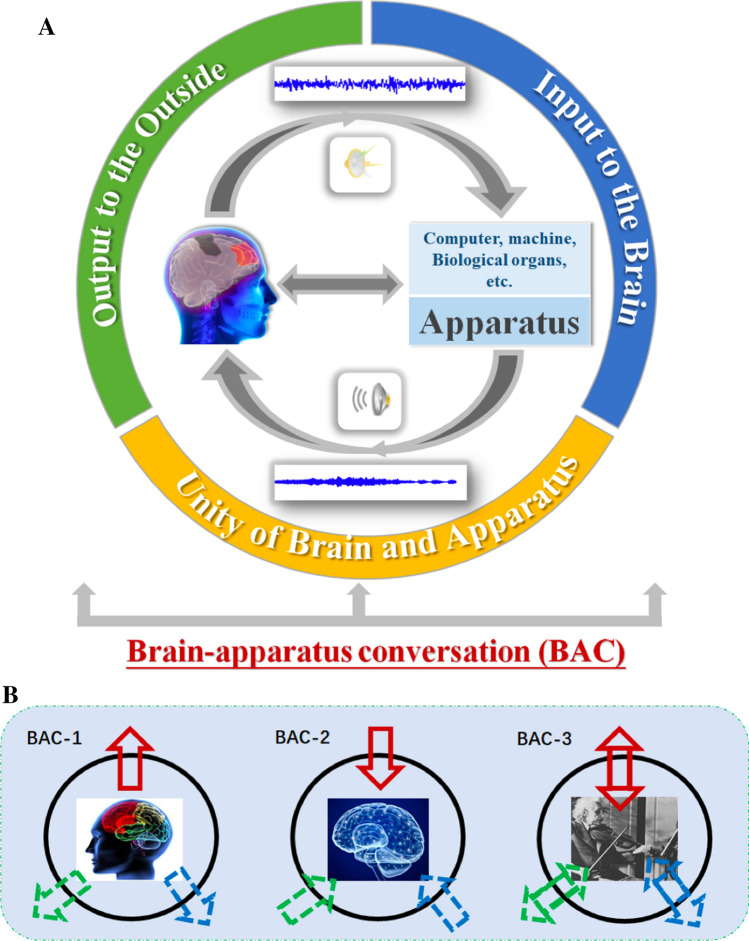
For a BAC system (Yao 2017), the brain is at the center. The brain’s interactions with the world outside itself can be divided into two categories. One category is the interaction with nonliving machines and the environment, and the other category is the interaction with animate machines or biological organs. Certainly, BAC can also be divided into “activating brain”, “modulating brain” and “brain enhancement” according to its functional goal (Yao 2017). In detail, BAC refers to information communication between the brain and a biological organ or a nonliving machine or environment (Fig. 1). Here, the word “apparatus” is adopted because the word refers to not just computers or machines (Vidal 1973), but it may also represent a biological organ of a living system (Kock 2005). The word “conversation” represents both one-way and bidirectional communication in nature. In this way, BAC can have another category focusing on information output (BAC-1), information input (BAC-2), and harmonious dialogue (BAC-3), respectively (Fig. 1).
Fig. 1.
Illustration of brain–apparatus conversation (BAC). a Left: brain functionalized with perception/execution, decision making, memory, consciousness, etc. Right: apparatus including both biological organs within a body and machine-like computers outside the body. Conversation covers unidirectional and bidirectional communication. b BAC-1 (the issue of output to the outside, releasing the brain): the color brain is normal, and the purpose is to functionally release the brain by repairing or reconstructing the biological output channels (i.e., blue arrow to inside organs such as the Gut and heart;green one to outside environment and tools by such as hand or artificial limb) or creating new channels (red one by neural signal decoding to control computer etc.) to make full use of the brain functions; BAC-2 (the issue of input to the brain, modulating brain): the dim brain is in disorder or needs to be enhanced, and the purpose is to utilize or repair the normal channels (i.e., blue one by such as medicine; green one by visual feedback or visual prostheses etc.) or create new channels (red one by encoded neural stimulus etc.) to intervene, repair or modulate brain function; and BAC-3 (the issue of unity of Brain and apparatus, developing brain): both the brain and the communication channels are normal, and the purpose is to enhance coordinated development between the brain and apparatus. (Color figure online)
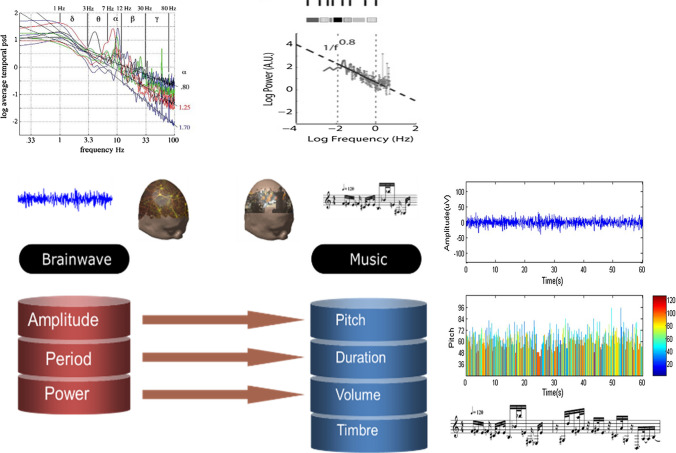
Regarding BAC-1 (the issue of output to the outside) in Fig. 1b, the color brain is intact and normal. The problem lies in the channels or the biological apparatus/organ outside the brain. The goal of the research is to functionally activate the brain by repairing the biological output channels or creating new channels to make full use of the brain functions by passing neural signals through the channel to interact/control something outside the brain. An example is the output to biological organs by the brain–spine interfaces in primates (Capogrosso et al. 2016). In the BAC-1, the brain is the base and active. The applications that create new output channels allow brain output independent of the biological pathways of peripheral nerves and muscles, which can be realized by directly reading out the neural signals, i.e., EEG, ECoG, LFP, neural spikes, or metabolic signals, i.e., fMRI, fNIRS, or EMG, EOG, to express the movement or other communication intent of the subject. Typical applications have included aiding paralyzed humans to communicate and interact with the outside world by invasive or noninvasive BCI technology (He et al. 2015; Mak and Wolpaw 2009; Miao et al. 2019). The most popular control signals used for BCI include sensorimotor rhythms (Ang and Guan 2017; Jin et al. 2019; Li et al. 2014; Liao et al. 2007a, b; Zhang et al. 2017), steady-state visual evoked potential (SSVEP) (Jiao et al. 2018; Maye et al. 2017; Yin et al. 2015; Zhang et al. 2010, 2018b), motion-onset visual evoked potential (mVEP) (Guo et al. 2008; Jin et al. 2012b; Ma et al. 2018), P300 (Jin et al. 2011, 2017; Long et al. 2011; Pan et al. 2013; Zhang et al. 2008), etc. Except for these BCI approaches, another interesting approach is the brainwave music developed in our laboratory (Fig. 2), where the power law followed by both music and EEG was utilized to generate scale-free brainwave music from EEG signals (Lu et al. 2012; Wu et al. 2009), and the resulting music may vividly indicate different states, such as rapid eye movement (REM) sleep, slow wave sleep (SWS) or wake states with eyes closed or open. Brainwave music moves the field forward by illustrating the scale-free physiological signals of the brain by a new auditory mode.
Fig. 2.
Scale-free music of the brain. The left panel shows the translation rules. Parameters of EEG, such as amplitude, period and power, are translated to the parameters of a note in music, such as pitch, duration, and volume, respectively. The timbre is arbitrarily defined as a piano or other instrument. The right panel is an illustration from EEG (up) to music MIDI (middle) and score (bottom)
(the right panel is reproduced with permission from Wu et al. 2009)
Regarding BAC-2 (the issue of input to the brain) in Fig. 1b, brain function is abnormal, and we need to utilize the normal channels or create new channels to intervene, repair or modulate brain function. In this category, the brain is passive. The approaches to repair/remold the brain functions by existing channels could be drug therapy (the digestive system and metabolic system), psychotherapy (auditory and visual channels), visual/auditory/tactile feedback treatment, etc. These conversations between the brain and the interactive object, such as drug, are realized through the biological channels and apparatus, which is different from the conventional BCI. For the drug therapy, a study showed that oxytocin may differentially act via the amygdala to enhance the salience of positive social attributes in women but the salience of negative attributes in men (Gao et al. 2016), implicitly indicating the potential of oxytocin for various mood/emotion disorders. For the psychotherapy, the approaches include auditory prostheses (cochlear implants) (Moore and Shannon 2009) and visual prostheses (Lovell et al. 2010) were used to repair the normal biological input channels,. For the feedback treatment, visual feedback has been used for the treatment of many chronic neurological disorders, such as phantom pain and hemiparesis from stroke (Ramachandran and Altschuler 2009; Walker et al. 2000), and various computer based cognitive therapies did similar work (Kendrick and Yao 2017). Another approach was the application of customized brainwave music. The researches demonstrated that the brainwave music can relieve orofacial pain (Huang et al. 2016), and the music exposure to Mozart K.448 was found to improve spatial cognition by enhancing brain-derived neurotrophic factor levels in dorsal hippocampal subregions of developing rats (Xing et al. 2016a), promote human subjects’ performance in the paper folding and cutting test and the pencil-and-paper maze test (Xing et al. 2016c). Another example is the Mozart music improved the clinical symptoms of schizophrenia (He et al. 2017; Yang et al. 2018) or reduced cognitive impairment in pilocarpine-induced status epilepticus rats (Xing et al. 2016b).
In general, the approaches to creating new input channels could be realized with invasive and noninvasive technologies. For example, DBS can be used for the treatment of Parkinson’s disease (Benabid et al. 2009). Other approaches include transcranial magnetic stimulation (TMS) (Si et al. 2018), transcranial direct-current stimulation (tDCS) (Geddes 2015), vagus nerve stimulation (VNS) (Englot et al. 2016), FES (Ang and Guan 2013; Ring and Weingarden 2007). A well-known example is to deliver electrical stimulation to rat whisker areas as a cue for left–right motions (Talwar et al. 2002). Recently, we found that electroconvulsive therapy (ECT) increased global functional connectivity density within the default mode network (DMN) in schizophrenia patients who were treatment resistant to pharmaceutical therapy (Huang et al. 2018; Jiang et al. 2019b).
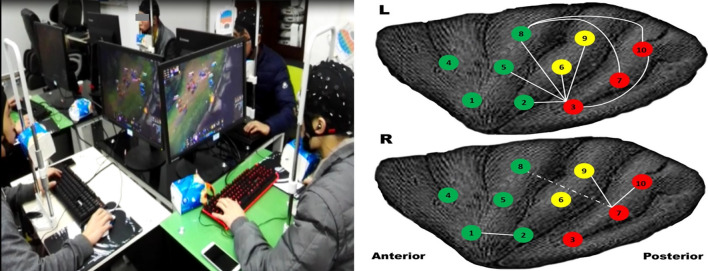
Regarding BAC-3 (the issue of unity of Brain and apparatus) in Fig. 1b, the starting point is to pursuit the unity of Brain and apparatus, and enhance the coordinated development between the brain and apparatus with the BAC channels. The harmony between brain and apparatus need to enhance or correct. By means of the bidirectional conversation between brain and apparatus, the collaborative capacity can be enhanced and specific functions can be reinforced. Because of the brain’s plasticity, training or repeatedly practicing can enhance the related brain function and even change our brain (Cotman and Berchtold 2002). In general, brain enhancements can be explicitly and specifically fostered by iteratively performing specific training tasks, such as music training (Gordon et al. 2015; Zatorre et al. 2007), physical training (Curlik and Shors 2013), cognitive task training (Taya et al. 2015), video gaming (Kuhn et al. 2014) and cyborg intelligence (Wu et al. 2016), etc. These trainings are usually interactively conducted with various information from apparatuses outside or inside the body to the brain, and the brain vividly make decision for further action. For example, in a virtual rehabilitation system, as shown in Fig. 3, which can be used to improve the motor rehabilitation of a paralyzed child after surgery. In this virtual rehabilitation system, the subject uses motor imagery paradigm to control the hand movement of the virtual man on the screen by EEG signals (output), and get feedback from the movement of virtual man on the screen (input). Regarding action video games (AVG), as shown in Fig. 4, it was found that gaming can enhance functional connectivity and alter structures in brain regions associated with attention and sensorimotor control (Gong et al. 2015). By examining insular subregions and their functional networks in AVG experts and amateurs, we found that gray matter volume in insular subregions changed, and the functional connectivity among the insular subregions, or between insular and attentional, sensorimotor areas was increased in experts compared with amateurs. In another related study, the findings showed that just 1-h AVG experience could improve the performance of nonexperts (Qiu et al. 2018). Meanwhile, it was also found that dancing might enhance the cortico-basal ganglia loops (Li et al. 2015, 2019). Obviously, the brain and the game process or the dace process are strongly interacted and mutually advancing.
Fig. 3.

An EEG based virtual rehabilitation training system. In this virtual rehabilitation system, the subject uses motor imagery paradigm to control the hand movement of the virtual man on the screen by EEG signals. The system is used to assist in the rehabilitation training of hand movement disorders, and can provide the real-time visual feedback to the subject
Fig. 4.
Interactive game. Left: video game scenario. Right: the enhanced links among different subregions of insula
(reproduced, with permission, from Gong et al. 2015)
In general, nourishment, exercise and learning are thought to be the three main factors that enhance our brain. Therefore, in addition to explicit conversations in the context of specific training/learning tasks, brain enhancement may be implicitly achieved by experience and environmental impact. During interactions in a wealthy society and environment, our cognitive, affective and learning skills might be enhanced during these interactions (Garthe et al. 2016; Grabinger and Dunlap 1995). Regarding brain development, stable and supportive social environments are crucial (McEwen and Gianaros 2010; Tost et al. 2015). Environmental factors can either promote or hinder brain health. For example, during development and aging, early maltreatment and lower socioeconomic environments can discordantly influence the structural and functional plasticity of the brain, which affect patterns of emotional expression and regulation, stress reactivity (McEwen and Gianaros 2010), etc. Meanwhile, exercise may induce hepatokines and adipokines to have beneficial impact on neurogenesis, cognitive function, appetite and metabolism, thus supporting the existence of a muscle–brain endocrine loop (Pedersen 2019). Furthermore, some cognitive enhancers (drugs) as treatments for neurodegenerative diseases and psychiatric disorders are being increasingly used by healthy individuals, although raising safety, ethical and regulatory concerns (Bruhl and Sahakian 2016; d’Angelo et al. 2017). It is the illegal use without scientific evidence support that may breakdown the harmony between the brain and the biological apparatus.
The main principles behind BAC
The above BAC involves seemingly disparate scenarios, so are there common cornerstones behind these approaches? In other words, what is the basic science behind these engineering issues? With these concerns, BAC is further updated as a new comprehensive cross area, Bacomics (BAC + omics). Here, we summarize five basic principles that appeared in the current BAC, which could guide BAC in the future.
The control principle
When the brain and an apparatus work together for a purpose, their conversations also act as feedforward and feedback from one to the other. Whether a message is considered as feedforward or feedback depends on the relative definition of the subject versus object. For a BCI system or an EEG-based neurofeedback system, the brain is usually taken as the subject, and the apparatus (computer/instrument actuator) could provide the visual/auditory/tactile feedback to the brain after a message is initially sent by the brain to the apparatus; this feedback can motivate the brain to initiate a new feedforward message to the apparatus. The various control principles in automatic control would be the cornerstones for a stable and valuable BAC system, where positive and negative feedback are the most essential elements. For example, a study showed that a system with feedback based on virtual reality techniques could improve feedback control, specifically for untrained subjects (Ron-Angevin and Díaz-Estrella 2009). Another study showed that visual feedback and somatosensory feedback were both effective for functional recovery from severe hemiplegia due to chronic stroke, but somatosensory feedback could be more effective for rehabilitation than visual feedback (Ono et al. 2014). In addition, a review paper concluded that there was a potential of BCI games with neurofeedback for children with the autism spectrum (Friedrich et al. 2014).
The harmonious principle
The pursuit of harmony is a fundamental drive in this world and a dream of humans. For BAC, harmony means the unity of brain and apparatus, and the coordinated development between the brain and apparatus by their conversations. The conversations will result in that the brain and an apparatus can enjoy and affect each other; otherwise, the conversation would be terminated or distorted. Interestingly, many studies have revealed that all sounds and their elements (pitch, melody, rhythm), natural images and geographical features that humans find enjoyable are composed of scale-free structures (Wu et al. 2015), which implies that the human brain may have adapted to the scale-free natural environment during evolution, runs in a scale-free manner in which healthy human body movements and brain activity (EEG and fMRI signals) all follow a scale-free pattern (Wu et al. 2009); this would mean that scale-free features, mathematically following the power law or Zipf’s law (Wu et al. 2015), would be an indicator of harmony. Based on this rule, we translated scalp EEGs into scale-free brainwave music (Wu et al. 2009), and the outcomes can vividly reflect intrinsic features of different brain states, such as REM, SWS, and eyes closed or eyes open wake states (Fig. 2) (Lu et al. 2012; Wu et al. 2013). Regardless, harmony would be one cornerstone of an effective conversation. The harmony or unity of brain and apparatus can be fostered by iteratively performing specific training tasks, such as mental and physical training (Li et al. 2015), maintaining the health of the body and mind (Sakaki et al. 2016; Shaffer et al. 2014) and exposure to supportive social environments (Di et al. 2019), etc.
The systems principle
As shown above, the brain, various human internal organs/apparatuses, and external devices/environments are integrated as a unified whole (Fig. 1). When this system becomes problematic, it may have stemmed from the organs or the conversation channels. Thus, for any problem of a BAC system, we need to systematically but not unilaterally investigate and explore the problems.
The principle of brain function plasticity
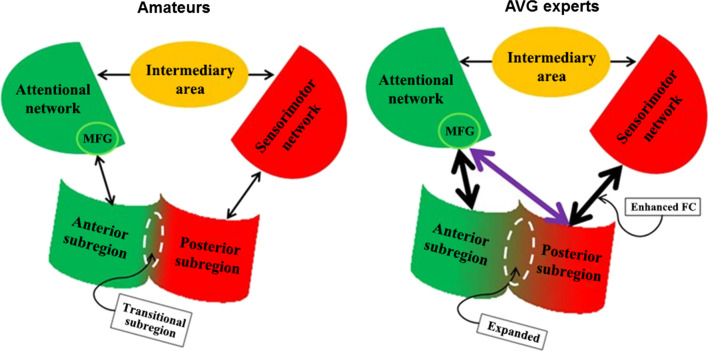
The brain is an inherently dynamic system that retains a capacity for plasticity and adaptive reorganization and can flexibly reconfigure interactions between spatially distributed networks. For example, previous studies have found that playing AVGs might enhance functionality and alter structures in brain regions associated with attention and sensorimotor control (Gong et al. 2015; Kendrick and Yao 2017) (Fig. 5), and a brain plasticity-based training program can be designed and used to enhance memory in healthy older adults (Mahncke et al. 2006). In addition, brain networks show dynamic functional segregation and integration during complex tasks (Shen et al. 2017), dynamic reconfiguration during learning (Bassett et al. 2011), etc. In summary, the conversation between brain and apparatus follows the basic principle of brain functional plasticity, so as to be helpful in understanding, protecting, nourishing, developing, and enhancing the brain, and not the other way around.
Fig. 5.
Brain plasticity in AVG experts. The shapes represent brain areas [middle frontal gyrus (MFG), anterior and posterior subregions of the insula]. The lines with two arrowheads represent functional connectivity (FC). Left: the pattern of the insular network in amateurs. Right: the pattern observed in the insular network in experts. Note that the right panel shows an expanded transitional subregion (the dotted line circle), a direct connection between the MFG and posterior subregion of the insula (purple line) and enhanced FC
(reproduced, with permission, from Gong et al. 2015). (Color figure online)
The principle of unity of opposites
Everything has two sides: amphetamine and methylphenidate could be used to treat ADHD in children and adolescents, but they have adverse effects when abused (Berman et al. 2009). Games can be used to improve performance, but gaming addiction has attracted great attention as a serious public and mental health issue (Weng et al. 2013). Similarly, not all music is beneficial as evidenced by Schoenberg music (Chamber symphony No. 2 Op. 38-I. Adagio) inducing a negative effect (Bates and Horvath 1971), and the retrograde version of Mozart K.448 and its rhythm having a negative cognitive effect that reduced spatial reasoning and memory capacity (Xing et al. 2016c). Another example is that thyroid hormone is essential for normal brain development, and either a deficit or an excess during development can have permanent effects on adult neurological function (Zoeller et al. 2002). These facts mean that our brain is very special, therefore, in addition to historical evidence confirming the effects of particular training, interventions or nutrients, any other new conversation needs to be evaluated carefully before application.
Opportunity, challenges, and future prospects
Opportunity and challenges
Research on BAC has experienced impressive growth in past decades, but along with the opportunities, significant scientific and technological challenges remain. One of the fundamental issues is the neural mechanisms behind BAC. As the brain is at the center of the BAC system, we need to know how the brain most effectively works and how this conversation influences the brain. In fact, BAC extends deep roots into basic neuroscience and becomes a powerful tool to investigate how neural circuits encode and decode information in real time and how this coding changes with physiological learning and plasticity (Moxon and Foffani 2015). For example, better knowledge of the neural mechanisms underpinning self-regulation will likely assist in the design of more efficient experimental protocols, tools and technologies for neurofeedback and in discovering more knowledge of neurophysiology (Sitaram et al. 2017). Understanding the mechanisms of individual differences in a BAC system would greatly hasten the effective application of BAC in various scenarios, and understanding the mechanisms from the brain network perspective could provide some new insight toward explaining individual differences (Gong et al. 2017; Li et al. 2016; Zhang et al. 2013a, b, 2015, 2016b). Furthermore, the signals used in BAC studies are modality dependent. For noninvasive studies, one or more techniques such as EEG, MEG, fMRI and NIRS can be adopted. For invasive studies, spikes, LFP, and ECoG may be adopted. The signals from different modalities could have different temporal–spatial resolutions. For noninvasive recording, EEG is the most popular because of the portability and low cost, but the recording electrodes still need to be optimized (e.g., convenient wear, removing the need for conductive gel) for the BAC system to go beyond the laboratory. For invasive recording, development of reliable and long-term recording devices and methods are still in the early stages (Hong and Lieber 2019). In the future, neural mechanism-based signal choice and utilization would be an important topic for an efficient BAC system.
In the framework of BAC, brainformatics methods are vital tools to decode neural activities (Yao 2017). Although current BAC may provide new channel for paralyzed people, it is slow when compared to natural behavior. To bridge the gap, new experimental paradigms and algorithms for Bacomics should be developed. For instances, in BAC-1, the paradigms that can evoke or induce fast, reliable and robust response will be promising, and recent efforts including face paradigm for P300 BAC (Jin et al. 2012a; Zhang et al. 2012) and joint frequency–phase modulation method for SSVEP BAC (Chen et al. 2015), etc. To decode neural activities, more powerful signal processing is desired, such as sparse Bayesian learning, extreme learning machine, deep learning, reinforcement leaning, and transfer learning, are anticipated to improve BAC system performance (Jin et al. 2018; Lawhern et al. 2018; Li et al. 2017; Lotte et al. 2007, 2018; Quitadamo et al. 2017; Sakhavi et al. 2018; Schirrmeister et al. 2017; Shanechi 2017; Zhang et al. 2017, 2018a). In EEG-based BAC systems, EEG signals are prone to interference by various noises, and the systems need to collect training samples for robust classifier training before online operation, which is time consuming (Jiao et al. 2019; Zerafa et al. 2018). One solution could be designing a powerful method based on machine learning and signal processing algorithms to exploit the intersubject information from available datasets (Jiao et al. 2019; Jin et al. 2020; Yuan et al. 2015). An alternative solution can resort to transfer learning which could be a useful option to reduce tedious calibration times (Jayaram et al. 2016; Nakanishi et al. 2019). Besides, another challenging and exciting BAC research could be the speech synthesis from human cortical activity, significant progress has been made with the help of deep learning methods (Anumanchipalli et al. 2019; Moses et al. 2019).
The diagnosis and intervention of brain diseases exacerbate societal and family burden. It is urgent to find efficient drugs and develop effective diagnostic and therapeutic approaches for brain disorders, neuropsychiatric disorders, and neurodegenerative brain diseases. Early intervention and modulation approaches are usually very important to halt or delay disease progression. The BAC has shown promise for the early intervention and modulation of a number of neurological and psychiatric disorders, and the methods included DBS, tDCS, neurofeedback (Benabid et al. 2009; Kuo et al. 2014; Ramot et al. 2017; Sitaram et al. 2017), and music and game. Apparently, BAC is not just a crucial and powerful tool to intervene and treat various brain disorders, it also opens new windows to understand the neural mechanisms underlying various dysfunctions.
In addition to applications in the treatment of brain disorders, BAC also opens an avenue for screening, cultivating and training people in specific professions. The BAC could provide more objective criteria for measurement and evaluation based on physiological measures. For example, BAC could be used with astronauts in their daily training, task execution and evaluation and with top athletes in their training for Olympic Games.
In this paper, we take the brain at the center of Bacomics, and definitely for specific organs, such as the gut, it is possible to use the conversation between the gut and brain to modulate the gut function by the brain. In this way, Bacomics covers an even larger domain to be explored as the organ to be modulated may be the gut, immune system, endocrine system, metabolic system, etc.
Finally, Bacomics entails ethical issues that are similar to other areas of neuroscience while raising special ethical considerations as well. Given that brain activating, modulating and enhancing technologies all touch the innermost parts of humans, there may be important ethical and social concerns to be considered, and these concerns should be investigated in a timely manner along the progress being made, to ensure that studies are being conducted under continuously updated rules of ethics.
Future prospects
Under the framework of BAC, many technologies are described in the above sections. Here, we specifically list several new directions that could be anticipated in the future (Fingelkurts and Fingelkurts 2018).
Neural tissue engineering and stem cell chimeras
A previous study has demonstrated the possibility to implant brain cells from a “more advanced” species to a “less advanced” species for augmenting brain function (Han et al. 2013). Han and colleagues implanted human astroglial cells into a mouse brain and discovered that the transplanted cell survived and the behavior and performance of the mouse in several evaluated tasks were enhanced.
Genetic brain–mind enhancement
Genetic manipulations could be another way leading to brain and cognitive enhancement, which has been demonstrated in rats and mice (Sandberg and Bostrom 2006). By genetic manipulations, the memory formation and retention of an adult mice were improved (Tang et al. 1999). Similarly, by increasing the amount of brain growth factors, memory and cognition was improved (Routtenberg et al. 2000). In addition, through pharmacological, dietary, or nutritional supplementation, gene modification can be achieved. For instance, children can gain cognitive enhancement when their mothers consume sufficient amounts of choline during pregnancy (Caudill et al. 2018).
Neural dust
The neural dust is an innovative technology that could provide an ultrasonic, low power solution for chronic brain–machine interfaces. The neural dust system consists of an external ultrasonic transceiver board that powers and communicates with a millimeter-scale implanted sensor (Seo et al. 2013, 2015, 2016). The neural dust could be placed throughout the brain and could remain in the brain for a lifetime, and it holds the potential for future bioelectronics-based therapies.
Human brain/cloud interface
Human knowledge has been accumulating at an accelerated exponential pace in the cloud, and it is impossible to keep pace with this increasingly rapid generation of human knowledge owing to our biologically constrained cognitive abilities. In the future, neural nanorobotics may provide a technology for creating a real-time system that allows the human brain to interface with the cloud (Martins et al. 2019). Such a system might allow the individuals to instantaneously access to all of the cumulative human knowledge available in the cloud, which could significantly improve human learning capacities and intelligence, etc.
Conclusion
The interfaces between the brain and the external world have shown tremendous advancements over the past decades. In the current paper, we provided a unified framework, i.e., brain–apparatus conversations (BAC), to further integrate the conversations between brain and various human internal organs and external instruments together. We further proposed Bacomics as a new comprehensive cross area and summarized some basic principles. In general, Bacomics open a much wider door for interdisciplinarity in the study of the brain, and the principles behind it further provide the general framework in which to realize or optimize various conversations. The potential opportunities and challenges coexist in the future.
Bacomics will bring potential benefits to the relevant research fields and practical applications. BAC not only opens its branches into many traditional neural engineering fields but also has become a powerful tool to investigate fundamental questions in neuroscience, intervene and treat various brain disorders and simultaneously open new windows to understand the neural mechanisms underlying various function and dysfunctions. The BAC also provides many specific avenues for screening, cultivating and training people in specific professions.
Author contributions
DY conceived and designed this study. YZ and DY wrote the paper. YZ, TL, PX, DG, JL, YX, CL, LD, YL, KC, JL, DY contributed to the BAC examples. All of the authors have read and approved the manuscript.
Funding
This study was financially supported by National Natural Science Foundation of China (81861128001, 81771925, 61761166001, 81571759), Project of Science and Technology Department of Sichuan Province (2017HH0001) and 111 project (B12027).
Footnotes
The original online version of this article was revised: The reference “Andrew A, Fingelkurts AAF (2018) After human. Futura 4:60–74.” Is corrected to “Fingelkurts AA, Fingelkurts AA (2018) After human. Futura.4:60–74.” and respective citation in the text is corrected from “Andrew and Fingelkurts 2018” to “Fingelkurts and Fingelkurts 2018”.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
8/10/2022
A Correction to this paper has been published: 10.1007/s11571-022-09853-8
References
- Abdulkader SN, Atia A, Mostafa M-SM. Brain computer interfacing: applications and challenges. Egypt Inform J. 2015;16:213–230. [Google Scholar]
- Agusti A, Garcia-Pardo MP, Lopez-Almela I, Campillo I, Maes M, Romani-Perez M, Sanz Y. Interplay between the gut–brain axis, obesity and cognitive function. Front Neurosci. 2018;12:155. doi: 10.3389/fnins.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajiboye AB, et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet. 2017;389:1821–1830. doi: 10.1016/S0140-6736(17)30601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang KK et al (2009) A clinical study of motor imagery-based brain-computer interface for upper limb robotic rehabilitation. In: Conference proceedings: annual international conference of the IEEE engineering in medicine and biology society, pp 5981–5984 [DOI] [PubMed]
- Ang KK, Guan C. Brain–computer interface in stroke rehabilitation. J Comput Sci Eng. 2013;7:139–146. [Google Scholar]
- Ang KK, Guan C. EEG-based strategies to detect motor imagery for control and rehabilitation. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2017;25:392–401. doi: 10.1109/TNSRE.2016.2646763. [DOI] [PubMed] [Google Scholar]
- Anguera JA, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumanchipalli GK, Chartier J, Chang EF. Speech synthesis from neural decoding of spoken sentences. Nature. 2019;568:493–498. doi: 10.1038/s41586-019-1119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arico P, Borghini G, Di Flumeri G, Sciaraffa N, Babiloni F. Passive BCI beyond the lab: current trends and future directions. Physiol Meas. 2018;39:08TR02. doi: 10.1088/1361-6579/aad57e. [DOI] [PubMed] [Google Scholar]
- Arns M, de Ridder S, Strehl U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin EEG Neurosci. 2009;40:180–189. doi: 10.1177/155005940904000311. [DOI] [PubMed] [Google Scholar]
- Bao C, et al. Effect of electro-acupuncture and moxibustion on brain connectivity in patients with Crohn’s disease: a resting-state fMRI study. Front Hum Neurosci. 2017;11:559. doi: 10.3389/fnhum.2017.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashivan P, Kar K, DiCarlo JJ. Neural population control via deep image synthesis. Science. 2019;364:eaav9436. doi: 10.1126/science.aav9436. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci USA. 2011;108:7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates FC, Horvath T. Discrimination learning with rhythmic and nonrhythmic background music. Percept Mot Skills. 1971;33:1123–1126. [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol Psychiatry. 2009;14:123–142. doi: 10.1038/mp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge R, Wilson S, Callaghan M, Coyle D. Neurogaming with motion-onset visual evoked potentials (mVEPs): adults versus teenagers. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2019;27:572–581. doi: 10.1109/TNSRE.2019.2904260. [DOI] [PubMed] [Google Scholar]
- Biasiucci A, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun. 2018;9:2421. doi: 10.1038/s41467-018-04673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer JA, Middlebrooks JC. Auditory cortical images of cochlear-implant stimuli: dependence on electrode configuration. J Neurophysiol. 2002;87:478–492. doi: 10.1152/jn.00212.2001. [DOI] [PubMed] [Google Scholar]
- Blankertz B, et al. The Berlin brain–computer interface: progress beyond communication and control. Front Neurosci. 2016;10:530. doi: 10.3389/fnins.2016.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota–gut–brain axis. Front Neurosci. 2018;12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton CE, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533:247–250. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- Bruhl AB, Sahakian BJ. Drugs, games, and devices for enhancing cognition: implications for work and society. Ann N Y Acad Sci. 2016;1369:195–217. doi: 10.1111/nyas.13040. [DOI] [PubMed] [Google Scholar]
- Capogrosso M, et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539:284–288. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catrambone V, Greco A, Vanello N, Scilingo EP, Valenza G. Time-resolved directional brain–heart interplay measurement through synthetic data generation models. Ann Biomed Eng. 2019;47:1479–1489. doi: 10.1007/s10439-019-02251-y. [DOI] [PubMed] [Google Scholar]
- Caudill MA, Strupp BJ, Muscalu L, Nevins JEH, Canfield RL. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: a randomized, double-blind, controlled feeding study. FASEB J Off Publ Fed Am Soc Exp Biol. 2018;32:2172–2180. doi: 10.1096/fj.201700692RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdo T, Ruiz A, Suarez A, Campoy C. Probiotic, prebiotic, and brain development. Nutrients. 2017;9:E1247. doi: 10.3390/nu9111247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Na J, Agres PF, Savalia NK, Park DC, Wig GS. Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proc Natl Acad Sci USA. 2018;115:E5144–E5153. doi: 10.1073/pnas.1714021115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary U, Birbaumer N, Ramos-Murguialday A. Brain–computer interfaces for communication and rehabilitation. Nat Rev Neurol. 2016;12:513–525. doi: 10.1038/nrneurol.2016.113. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang Y, Nakanishi M, Gao X, Jung TP, Gao S. High-speed spelling with a noninvasive brain–computer interface. Proc Natl Acad Sci USA. 2015;112:E6058–E6067. doi: 10.1073/pnas.1508080112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Teng L, Che-Jui C, Bor-Shyh L, Shao-Hang H, Chih-Feng C, Wang IJ. A real-time wireless brain–computer interface system for drowsiness detection. IEEE Trans Biomed Circuits Syst. 2010;4:214–222. doi: 10.1109/TBCAS.2010.2046415. [DOI] [PubMed] [Google Scholar]
- Cincotti F, et al. Non-invasive brain–computer interface system: towards its application as assistive technology. Brain Res Bull. 2008;75:796–803. doi: 10.1016/j.brainresbull.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinel C, Valeriani D, Poli R. Neurotechnologies for human cognitive augmentation: current state of the art and future prospects. Front Hum Neurosci. 2019;13:13. doi: 10.3389/fnhum.2019.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan CG, He B. Brain–computer interface control in a virtual reality environment and applications for the internet of things. IEEE Access. 2018;6:10840–10849. doi: 10.1109/ACCESS.2018.2809453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Curlik DM, 2nd, Shors TJ. Training your brain: do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus? Neuropharmacology. 2013;64:506–514. doi: 10.1016/j.neuropharm.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JJ, Wolpaw JR. Brain–computer interfaces in neurological rehabilitation. Lancet Neurol. 2008;7:1032–1043. doi: 10.1016/S1474-4422(08)70223-0. [DOI] [PubMed] [Google Scholar]
- d’Angelo L, Camilla S, Savulich G, Sahakian BJ. Lifestyle use of drugs by healthy people for enhancing cognition, creativity, motivation and pleasure. Br J Pharmacol. 2017;174:3257–3267. doi: 10.1111/bph.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- Di X, Wolfer M, Kühn S, Zhang Z, Biswal BB (2019) Estimations of the weather effects on brain functions using functional MRI—a cautionary tale. bioRxiv:646695 [DOI] [PMC free article] [PubMed]
- Diaz Heijtz R, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrakopoulos GN, et al. Functional connectivity analysis of mental fatigue reveals different network topological alterations between driving and vigilance tasks. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2018;26:740–749. doi: 10.1109/TNSRE.2018.2791936. [DOI] [PubMed] [Google Scholar]
- Do AH, Wang PT, King CE, Abiri A, Nenadic Z. Brain–computer interface controlled functional electrical stimulation system for ankle movement. J Neuroeng Rehabil. 2011;8:49. doi: 10.1186/1743-0003-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP. Connecting cortex to machines: recent advances in brain interfaces. Nat Neurosci. 2002;5(Suppl):1085–1088. doi: 10.1038/nn947. [DOI] [PubMed] [Google Scholar]
- Donoghue JP. Bridging the brain to the world: a perspective on neural interface systems. Neuron. 2008;60:511–521. doi: 10.1016/j.neuron.2008.10.037. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery. 2016;79:345–353. doi: 10.1227/NEU.0000000000001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun-Sun J, et al. Effect of acupuncture on patients with mild cognitive impairment assessed using functional near-infrared spectroscopy on week 12 (close-out): a pilot study protocol. Integr Med Res. 2018;7:287–295. doi: 10.1016/j.imr.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faes L, Nollo G, Jurysta F, Marinazzo D. Information dynamics of brain–heart physiological networks during sleep. New J Phys. 2014;16:105005. [Google Scholar]
- Faes L, Marinazzo D, Jurysta F, Nollo G. Linear and non-linear brain–heart and brain–brain interactions during sleep. Physiol Meas. 2015;36:683–698. doi: 10.1088/0967-3334/36/4/683. [DOI] [PubMed] [Google Scholar]
- Fan J, Wade JW, Key AP, Warren ZE, Sarkar N. EEG-based affect and workload recognition in a virtual driving environment for ASD intervention. IEEE Trans Biomed Eng. 2018;65:43–51. doi: 10.1109/TBME.2017.2693157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. After human. Futura. 2018;4:60–74. [Google Scholar]
- Foster JA, McVey Neufeld KA. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Franceschini S, Gori S, Ruffino M, Viola S, Molteni M, Facoetti A. Action video games make dyslexic children read better. Curr Biol. 2013;23:462–466. doi: 10.1016/j.cub.2013.01.044. [DOI] [PubMed] [Google Scholar]
- Fregni F, et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res. 2005;166:23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- Friedrich EV, Suttie N, Sivanathan A, Lim T, Louchart S, Pineda JA. Brain–computer interface game applications for combined neurofeedback and biofeedback treatment for children on the autism spectrum. Front Neuroeng. 2014;7:21. doi: 10.3389/fneng.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoli A, Loconsole C, Leonardis D, Banno F, Barsotti M, Chisari C, Bergamasco M. A new gaze-BCI-driven control of an upper limb exoskeleton for rehabilitation in real-world tasks. IEEE Trans Syst Man Cybern Part C (Appl Rev) 2012;42:1169–1179. [Google Scholar]
- Gao S, et al. Oxytocin, the peptide that bonds the sexes also divides them. Proc Natl Acad Sci USA. 2016;113:7650–7654. doi: 10.1073/pnas.1602620113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Roeder I, Kempermann G. Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus. 2016;26:261–271. doi: 10.1002/hipo.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateau T, Durantin G, Lancelot F, Scannella S, Dehais F. Real-time state estimation in a flight simulator using fNIRS. PLoS ONE. 2015;10:e0121279. doi: 10.1371/journal.pone.0121279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gateau T, Ayaz H, Dehais F. In silico vs. over the clouds: on-the-fly mental state estimation of aircraft pilots, using a functional near infrared spectroscopy based passive-BCI. Front Hum Neurosci. 2018;12:187. doi: 10.3389/fnhum.2018.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes L. Brain stimulation in children spurs hope—and concern. Nature. 2015;525:436–437. doi: 10.1038/525436a. [DOI] [PubMed] [Google Scholar]
- George MS, et al. Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiat. 2000;47:287–295. doi: 10.1016/s0006-3223(99)00308-x. [DOI] [PubMed] [Google Scholar]
- Gharabaghi A, et al. Coupling brain–machine interfaces with cortical stimulation for brain-state dependent stimulation: enhancing motor cortex excitability for neurorehabilitation. Front Hum Neurosci. 2014;8:122. doi: 10.3389/fnhum.2014.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilja V, Chestek CA, Diester I, Henderson JM, Deisseroth K, Shenoy KV. Challenges and opportunities for next-generation intracortically based neural prostheses. IEEE Trans Biomed Eng. 2011;58:1891–1899. doi: 10.1109/TBME.2011.2107553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, He H, Liu D, Ma W, Dong L, Luo C, Yao D. Enhanced functional connectivity and increased gray matter volume of insula related to action video game playing. Sci Rep. 2015;5:9763. doi: 10.1038/srep09763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, et al. White matter connectivity pattern associate with characteristics of scalp EEG signals. Brain Topogr. 2017;30:797–809. doi: 10.1007/s10548-017-0581-z. [DOI] [PubMed] [Google Scholar]
- Gordon RL, Fehd HM, McCandliss BD. Does music training enhance literacy skills? A meta-analysis. Front Psychol. 2015;6:1777. doi: 10.3389/fpsyg.2015.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, et al. Defining optimal brain health in adults: a presidential advisory from the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. doi: 10.1161/STR.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabinger RS, Dunlap JC. Rich environments for active learning: a definition. ALT J. 1995;3:5–34. [Google Scholar]
- Graf AB, Andersen RA. Brain–machine interface for eye movements. Proc Natl Acad Sci USA. 2014;111:17630–17635. doi: 10.1073/pnas.1419977111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau C, et al. Conscious brain-to-brain communication in humans using non-invasive technologies. PLoS ONE. 2014;9:e105225. doi: 10.1371/journal.pone.0105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N, et al. Noninvasive deep brain stimulation via temporally interfering electric fields. Cell. 2017;169(1029–1041):e1016. doi: 10.1016/j.cell.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Hong B, Gao X, Gao S. A brain–computer interface using motion-onset visual evoked potential. J Neural Eng. 2008;5:477–485. doi: 10.1088/1741-2560/5/4/011. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Han X, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsopoulos NG, Donoghue JP. The science of neural interface systems. Annu Rev Neurosci. 2009;32:249–266. doi: 10.1146/annurev.neuro.051508.135241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Baxter B, Edelman BJ, Cline CC, Wenjing WY. Noninvasive brain–computer interfaces based on sensorimotor rhythms. Proc IEEE. 2015;103:907–925. doi: 10.1109/jproc.2015.2407272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, et al. Music intervention leads to increased insular connectivity and improved clinical symptoms in schizophrenia. Front Neurosci. 2017;11:744. doi: 10.3389/fnins.2017.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde S. Music-based cognitive remediation therapy for patients with traumatic brain injury. Front Neurol. 2014;5:34. doi: 10.3389/fneur.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron JA, Thompson MC, Brown T, Chizeck HJ, Ojemann JG, Ko AL. Cortical brain–computer interface for closed-loop deep brain stimulation. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2017;25:2180–2187. doi: 10.1109/TNSRE.2017.2705661. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G, Lieber CM. Novel electrode technologies for neural recordings. Nat Rev Neurosci. 2019;20:330–345. doi: 10.1038/s41583-019-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Hu Y, Li X, Pan Y, Cheng X. Brain-to-brain synchronization across two persons predicts mutual prosociality. Soc Cogn Affect Neurosci. 2017;12:1835–1844. doi: 10.1093/scan/nsx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, et al. The effects of customised brainwave music on orofacial pain induced by orthodontic tooth movement. Oral Dis. 2016;22:766–774. doi: 10.1111/odi.12542. [DOI] [PubMed] [Google Scholar]
- Huang H, et al. Increased resting-state global functional connectivity density of default mode network in schizophrenia subjects treated with electroconvulsive therapy. Schizophr Res. 2018;197:192–199. doi: 10.1016/j.schres.2017.10.044. [DOI] [PubMed] [Google Scholar]
- Iaccarino HF, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540:230–235. doi: 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman CS, et al. Direct electrical stimulation of the amygdala enhances declarative memory in humans. Proc Natl Acad Sci USA. 2018;115:98–103. doi: 10.1073/pnas.1714058114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram V, Alamgir M, Altun Y, Scholkopf B, Grosse-Wentrup M. Transfer learning in brain–computer interfaces. IEEE Comput Intell Mag. 2016;11:20–31. [Google Scholar]
- Jensen O, Bahramisharif A, Oostenveld R, Klanke S, Hadjipapas A, Okazaki YO, van Gerven MA. Using brain–computer interfaces and brain-state dependent stimulation as tools in cognitive neuroscience. Front Psychol. 2011;2:100. doi: 10.3389/fpsyg.2011.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Stocco A, Losey DM, Abernethy JA, Prat CS, Rao RP. BrainNet: a multi-person brain-to-brain interface for direct collaboration between brains. Sci Rep. 2019;9:6115. doi: 10.1038/s41598-019-41895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, et al. Insular changes induced by electroconvulsive therapy response to symptom improvements in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:254–262. doi: 10.1016/j.pnpbp.2018.09.009. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Zhang Y, Wang Y, Wang B, Jin J, Wang X. A novel multilayer correlation maximization model for improving CCA-based frequency recognition in SSVEP brain–computer interface. Int J Neural Syst. 2018;28:1750039. doi: 10.1142/S0129065717500393. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Zhang Y, Chen X, Yin E, Jin J, Wang X, Cichocki A. Sparse group representation model for motor imagery EEG classification. IEEE J Biomed Health Inform. 2019;23:631–641. doi: 10.1109/JBHI.2018.2832538. [DOI] [PubMed] [Google Scholar]
- Jin J, Allison BZ, Sellers EW, Brunner C, Horki P, Wang X, Neuper C. An adaptive P300-based control system. J Neural Eng. 2011;8:036006. doi: 10.1088/1741-2560/8/3/036006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Allison BZ, Kaufmann T, Kubler A, Zhang Y, Wang X, Cichocki A. The changing face of P300 BCIs: a comparison of stimulus changes in a P300 BCI involving faces, emotion, and movement. PLoS ONE. 2012;7:e49688. doi: 10.1371/journal.pone.0049688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Allison BZ, Wang X, Neuper C. A combined brain–computer interface based on P300 potentials and motion-onset visual evoked potentials. J Neurosci Methods. 2012;205:265–276. doi: 10.1016/j.jneumeth.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Jin J, Zhang H, Daly I, Wang X, Cichocki A. An improved P300 pattern in BCI to catch user’s attention. J Neural Eng. 2017;14:036001. doi: 10.1088/1741-2552/aa6213. [DOI] [PubMed] [Google Scholar]
- Jin Z, Zhou G, Gao D, Zhang Y. EEG classification using sparse Bayesian extreme learning machine for brain–computer interface. Neural Comput Appl. 2018 doi: 10.1007/s00521-018-3735-3. [DOI] [Google Scholar]
- Jin J, Miao Y, Daly I, Zuo C, Hu D, Cichocki A. Correlation-based channel selection and regularized feature optimization for MI-based BCI. Neural Netw Off J Int Neural Netw Soc. 2019;118:262–270. doi: 10.1016/j.neunet.2019.07.008. [DOI] [PubMed] [Google Scholar]
- Jin J, Li S, Daly I, Miao Y, Liu C, Wang X, Cichocki A. The study of generic model set for reducing calibration time in P300-based brain–computer interface. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2020;28:3–12. doi: 10.1109/TNSRE.2019.2956488. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Yao D. Can computer-based cognitive therapy become a front-line option for prevention and treatment of mental disorders? Am J Psychiatry. 2017;174:303–304. doi: 10.1176/appi.ajp.2017.16121439. [DOI] [PubMed] [Google Scholar]
- Khushaba RN, Wise C, Kodagoda S, Louviere J, Kahn BE, Townsend C. Consumer neuroscience: assessing the brain response to marketing stimuli using electroencephalogram (EEG) and eye tracking. Expert Syst Appl. 2013;40:3803–3812. [Google Scholar]
- Kim P, et al. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc Natl Acad Sci USA. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock N. Media richness or media naturalness? The evolution of our biological communication apparatus and its influence on our behavior toward e-communication tools. IEEE Trans Prof Commun. 2005;48:117–130. [Google Scholar]
- Krusienski DJ, Sellers EW, McFarland DJ, Vaughan TM, Wolpaw JR. Toward enhanced P300 speller performance. J Neurosci Methods. 2008;167:15–21. doi: 10.1016/j.jneumeth.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler A, Mushahwar VK, Hochberg LR, Donoghue JP. BCI meeting 2005—workshop on clinical issues and applications. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2006;14:131–134. doi: 10.1109/tnsre.2006.875585. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gleich T, Lorenz RC, Lindenberger U, Gallinat J. Playing Super Mario induces structural brain plasticity: gray matter changes resulting from training with a commercial video game. Mol Psychiatry. 2014;19:265–271. doi: 10.1038/mp.2013.120. [DOI] [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage. 2014;85(Pt 3):948–960. doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- Lawhern VJ, Solon AJ, Waytowich NR, Gordon SM, Hung CP, Lance BJ. EEGNet: a compact convolutional neural network for EEG-based brain–computer interfaces. J Neural Eng. 2018;15:056013. doi: 10.1088/1741-2552/aace8c. [DOI] [PubMed] [Google Scholar]
- Lee TS, et al. A brain–computer interface based cognitive training system for healthy elderly: a randomized control pilot study for usability and preliminary efficacy. PLoS ONE. 2013;8:e79419. doi: 10.1371/journal.pone.0079419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kim S, Kim B, Lee C, Chung YA, Kim L, Yoo SS. Non-invasive transmission of sensorimotor information in humans using an EEG/focused ultrasound brain-to-brain interface. PLoS ONE. 2017;12:e0178476. doi: 10.1371/journal.pone.0178476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang D. Brain–computer interface controlled cyborg: establishing a functional information transfer pathway from human brain to cockroach brain. PLoS ONE. 2016;11:e0150667. doi: 10.1371/journal.pone.0150667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Long J, Yu T, Yu Z, Wang C, Zhang H, Guan C. An EEG-based BCI system for 2-D cursor control by combining Mu/Beta rhythm and P300 potential. IEEE Trans Biomed Eng. 2010;57:2495–2505. doi: 10.1109/TBME.2010.2055564. [DOI] [PubMed] [Google Scholar]
- Li X, Guan C, Zhang H, Ang KK, Ong SH. Adaptation of motor imagery EEG classification model based on tensor decomposition. J Neural Eng. 2014;11:056020. doi: 10.1088/1741-2560/11/5/056020. [DOI] [PubMed] [Google Scholar]
- Li G, et al. Identifying enhanced cortico-basal ganglia loops associated with prolonged dance training. Sci Rep. 2015;5:10271. doi: 10.1038/srep10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, et al. The time-varying networks in P300: a task-evoked EEG study. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2016;24:725–733. doi: 10.1109/TNSRE.2016.2523678. [DOI] [PubMed] [Google Scholar]
- Li X, Guan C, Zhang H, Ang KK. A unified Fisher’s ratio learning method for spatial filter optimization. IEEE Trans Neural Netw Learn Syst. 2017;28:2727–2737. doi: 10.1109/TNNLS.2016.2601084. [DOI] [PubMed] [Google Scholar]
- Li G, et al. Increased insular connectivity and enhanced empathic ability associated with dance/music training. Neural Plast. 2019;2019:9693109. doi: 10.1155/2019/9693109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Yao D, Li C. Transductive SVM for reducing the training effort in BCI. J Neural Eng. 2007;4:246–254. doi: 10.1088/1741-2560/4/3/010. [DOI] [PubMed] [Google Scholar]
- Liao X, Yao D, Wu D, Li C. Combining spatial filters for the classification of single-trial EEG in a finger movement task. IEEE Trans Biomed Eng. 2007;54:821–831. doi: 10.1109/TBME.2006.889206. [DOI] [PubMed] [Google Scholar]
- Lim CG, et al. A brain–computer interface based attention training program for treating attention deficit hyperactivity disorder. PLoS ONE. 2012;7:e46692. doi: 10.1371/journal.pone.0046692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, et al. Responsive neurostimulation for the treatment of medically intractable epilepsy. Brain Res Bull. 2013;97:39–47. doi: 10.1016/j.brainresbull.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Liu P, et al. Altered topological patterns of brain functional networks in Crohn’s disease. Brain Imaging Behav. 2018;12:1466–1478. doi: 10.1007/s11682-017-9814-8. [DOI] [PubMed] [Google Scholar]
- Long J, Gu Z, Li Y, Yu T, Li F, Fu M. Semi-supervised joint spatio-temporal feature selection for P300-based BCI speller. Cogn Neurodyn. 2011;5:387–398. doi: 10.1007/s11571-011-9167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Li Y, Wang H, Yu T, Pan J, Li F. A hybrid brain computer interface to control the direction and speed of a simulated or real wheelchair. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2012;20:720–729. doi: 10.1109/TNSRE.2012.2197221. [DOI] [PubMed] [Google Scholar]
- Lopez-Larraz E, et al. Control of an ambulatory exoskeleton with a brain–machine interface for spinal cord injury gait rehabilitation. Front Neurosci. 2016;10:359. doi: 10.3389/fnins.2016.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotte F, Congedo M, Lecuyer A, Lamarche F, Arnaldi B. A review of classification algorithms for EEG-based brain–computer interfaces. J Neural Eng. 2007;4:R1–R13. doi: 10.1088/1741-2560/4/2/R01. [DOI] [PubMed] [Google Scholar]
- Lotte F, Bougrain L, Cichocki A, Clerc M, Congedo M, Rakotomamonjy A, Yger F. A review of classification algorithms for EEG-based brain–computer interfaces: a 10 year update. J Neural Eng. 2018;15:031005. doi: 10.1088/1741-2552/aab2f2. [DOI] [PubMed] [Google Scholar]
- Lovell NH, Morley JW, Chen SC, Hallum LE, Suaning GJ. Biological–machine systems integration: engineering the neural interface. Proc IEEE. 2010;98:418–431. [Google Scholar]
- Lu J, Wu D, Yang H, Luo C, Li C, Yao D. Scale-free brain-wave music from simultaneously EEG and fMRI recordings. PLoS ONE. 2012;7:e49773. doi: 10.1371/journal.pone.0049773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubianiker N, et al. Process-based framework for precise neuromodulation. Nat Hum Behav. 2019;3:436–445. doi: 10.1038/s41562-019-0573-y. [DOI] [PubMed] [Google Scholar]
- Luu TP, He Y, Brown S, Nakagame S, Contreras-Vidal JL. Gait adaptation to visual kinematic perturbations using a real-time closed-loop brain–computer interface to a virtual reality avatar. J Neural Eng. 2016;13:036006. doi: 10.1088/1741-2560/13/3/036006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Li F, Li P, Yao D, Zhang Y, Xu P. An adaptive calibration framework for mVEP-based brain–computer interface. Comput Math Methods Med. 2018;2018:9476432. doi: 10.1155/2018/9476432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahncke HW, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci USA. 2006;103:12523–12528. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak JN, Wolpaw JR. Clinical applications of brain–computer interfaces: current state and future prospects. IEEE Rev Biomed Eng. 2009;2:187–199. doi: 10.1109/RBME.2009.2035356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins NRB, et al. Human brain/cloud interface. Front Neurosci. 2019;13:112. doi: 10.3389/fnins.2019.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maye A, Zhang D, Engel AK. Utilizing retinotopic mapping for a multi-target SSVEP BCI with a single flicker frequency. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2017;25:1026–1036. doi: 10.1109/TNSRE.2017.2666479. [DOI] [PubMed] [Google Scholar]
- Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DJ, Daly J, Boulay C, Parvaz M. Therapeutic applications of BCI technologies. Brain Comput Interfaces. 2017;47:37–52. doi: 10.1080/2326263X.2017.1307625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15:663–668. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- Miao Y, et al. An ERP-based BCI with peripheral stimuli: validation with ALS patients. Cogn Neurodyn. 2019;14:1–13. doi: 10.1007/s11571-019-09541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min BK, Marzelli MJ, Yoo SS. Neuroimaging-based approaches in the brain–computer interface. Trends Biotechnol. 2010;28:552–560. doi: 10.1016/j.tibtech.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Moldoveanu A, Ferche O-M, Moldoveanu F, Lupu RG, Cinteză D, Irimia DC, Toader C. The TRAVEE system for a multimodal neuromotor rehabilitation. IEEE Access. 2019;7:8151–8171. [Google Scholar]
- Moore DR, Shannon RV. Beyond cochlear implants: awakening the deafened brain. Nat Neurosci. 2009;12:686–691. doi: 10.1038/nn.2326. [DOI] [PubMed] [Google Scholar]
- Moses DA, Leonard MK, Makin JG, Chang EF. Real-time decoding of question-and-answer speech dialogue using human cortical activity. Nat Commun. 2019;10:3096. doi: 10.1038/s41467-019-10994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon KA, Foffani G. Brain–machine interfaces beyond neuroprosthetics. Neuron. 2015;86:55–67. doi: 10.1016/j.neuron.2015.03.036. [DOI] [PubMed] [Google Scholar]
- Muller-Putz GR, Pfurtscheller G. Control of an electrical prosthesis with an SSVEP-based BCI. IEEE Trans Biomed Eng. 2008;55:361–364. doi: 10.1109/TBME.2007.897815. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Wang YT, Wei CS, Chiang KJ, Jung TP. Facilitating calibration in high-speed BCI spellers via leveraging cross-device shared latent responses. IEEE Trans Biomed Eng. 2019 doi: 10.1109/TBME.2019.2929745. [DOI] [PubMed] [Google Scholar]
- Nicolas-Alonso LF, Gomez-Gil J. Brain computer interfaces, a review. Sensors. 2012;12:1211–1279. doi: 10.3390/s120201211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol. 2009;219:14–19. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouchi R, et al. Brain training game improves executive functions and processing speed in the elderly: a randomized controlled trial. PLoS ONE. 2012;7:e29676. doi: 10.1371/journal.pone.0029676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JE, Lebedev MA, Ifft PJ, Zhuang KZ, Shokur S, Bleuler H, Nicolelis MA. Active tactile exploration using a brain–machine–brain interface. Nature. 2011;479:228. doi: 10.1038/nature10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, et al. Brain–computer interface with somatosensory feedback improves functional recovery from severe hemiplegia due to chronic stroke. Front Neuroeng. 2014;7:19. doi: 10.3389/fneng.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais-Vieira M, Chiuffa G, Lebedev M, Yadav A, Nicolelis MA. Building an organic computing device with multiple interconnected brains. Sci Rep. 2015;5:11869. doi: 10.1038/srep11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Li Y, Gu Z, Yu Z. A comparison study of two P300 speller paradigms for brain–computer interface. Cogn Neurodyn. 2013;7:523–529. doi: 10.1007/s11571-013-9253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Patron E, Mennella R, Messerotti Benvenuti S, Thayer JF. The frontal cortex is a heart-brake: reduction in delta oscillations is associated with heart rate deceleration. NeuroImage. 2019;188:403–410. doi: 10.1016/j.neuroimage.2018.12.035. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. Physical activity and muscle–brain crosstalk. Nat Rev Endocrinol. 2019;15:383–392. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- Pereira VH, Cerqueira JJ, Palha JA, Sousa N. Stressed brain, diseased heart: a review on the pathophysiologic mechanisms of neurocardiology. Int J Cardiol. 2013;166:30–37. doi: 10.1016/j.ijcard.2012.03.165. [DOI] [PubMed] [Google Scholar]
- Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9:336–346. doi: 10.1016/j.brs.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Muller GR, Pfurtscheller J, Gerner HJ, Rupp R. ‘Thought’—control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett. 2003;351:33–36. doi: 10.1016/s0304-3940(03)00947-9. [DOI] [PubMed] [Google Scholar]
- Ponce CR, Xiao W, Schade PF, Hartmann TS, Kreiman G, Livingstone MS. Evolving images for visual neurons using a deep generative network reveals coding principles and neuronal preferences. Cell. 2019;177(999–1009):e1010. doi: 10.1016/j.cell.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu N, et al. Rapid improvement in visual selective attention related to action video gaming experience. Front Hum Neurosci. 2018;12:47. doi: 10.3389/fnhum.2018.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitadamo LR, Cavrini F, Sbernini L, Riillo F, Bianchi L, Seri S, Saggio G. Support vector machines to detect physiological patterns for EEG and EMG-based human–computer interaction: a review. J Neural Eng. 2017;14:011001. doi: 10.1088/1741-2552/14/1/011001. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain J Neurol. 2009;132:1693–1710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- Ramadan RA, Vasilakos AV. Brain computer interface: control signals review. Neurocomputing. 2017;223:26–44. [Google Scholar]
- Ramot M, et al. Direct modulation of aberrant brain network connectivity through real-time neurofeedback. eLife. 2017;6:e28974. doi: 10.7554/eLife.28974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP. Brain–computer interfacing: an introduction. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- Rao RP. Towards neural co-processors for the brain: combining decoding and encoding in brain–computer interfaces. Curr Opin Neurobiol. 2019;55:142–151. doi: 10.1016/j.conb.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP, Stocco A, Bryan M, Sarma D, Youngquist TM, Wu J, Prat CS. A direct brain-to-brain interface in humans. PLoS ONE. 2014;9:e111332. doi: 10.1371/journal.pone.0111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsamen B, et al. Controlling a wheelchair indoors using thought. IEEE Intell Syst. 2007;22:18–24. [Google Scholar]
- Rebsamen B, Guan C, Zhang H, Wang C, Teo C, Ang MH, Jr, Burdet E. A brain controlled wheelchair to navigate in familiar environments. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2010;18:590–598. doi: 10.1109/TNSRE.2010.2049862. [DOI] [PubMed] [Google Scholar]
- Reinhart RMG, Nguyen JA. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci. 2019;22:820–827. doi: 10.1038/s41593-019-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring H, Weingarden H. Neuromodulation by functional electrical stimulation (FES) of limb paralysis after stroke. Acta Neurochir Suppl. 2007;97:375–380. doi: 10.1007/978-3-211-33079-1_49. [DOI] [PubMed] [Google Scholar]
- Ron-Angevin R, Díaz-Estrella A. Brain–computer interface: changes in performance using virtual reality techniques. Neurosci Lett. 2009;449:123–127. doi: 10.1016/j.neulet.2008.10.099. [DOI] [PubMed] [Google Scholar]
- Rong P, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172–179. doi: 10.1016/j.jad.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A, Cantallops I, Zaffuto S, Serrano P, Namgung U. Enhanced learning after genetic overexpression of a brain growth protein. Proc Natl Acad Sci USA. 2000;97:7657–7662. doi: 10.1073/pnas.97.13.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Yoo HJ, Nga L, Lee TH, Thayer JF, Mather M. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. NeuroImage. 2016;139:44–52. doi: 10.1016/j.neuroimage.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhavi S, Guan C, Yan S. Learning temporal information for brain–computer interface using convolutional neural networks. IEEE Trans Neural Netw Learn Syst. 2018;29:5619–5629. doi: 10.1109/TNNLS.2018.2789927. [DOI] [PubMed] [Google Scholar]
- Salvaris M, Sepulveda F. Visual modifications on the P300 speller BCI paradigm. J Neural Eng. 2009;6:046011. doi: 10.1088/1741-2560/6/4/046011. [DOI] [PubMed] [Google Scholar]
- Samuels MA. The brain–heart connection. Circulation. 2007;116:77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- Sandberg A, Bostrom N. Converging cognitive enhancements. Ann N Y Acad Sci. 2006;1093:201–227. doi: 10.1196/annals.1382.015. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and the brain: individual variability and the inverted-U. Nat Neurosci. 2015;18:1344–1346. doi: 10.1038/nn.4109. [DOI] [PubMed] [Google Scholar]
- Schirrmeister RT, et al. Deep learning with convolutional neural networks for EEG decoding and visualization. Hum Brain Mapp. 2017;38:5391–5420. doi: 10.1002/hbm.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AB, Cui XT, Weber DJ, Moran DW. Brain-controlled interfaces: movement restoration with neural prosthetics. Neuron. 2006;52:205–220. doi: 10.1016/j.neuron.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Seo D, Carmena JM, Rabaey JM, Alon E, Maharbiz MM (2013) Neural dust: an ultrasonic, low power solution for chronic brain–machine interfaces. arXiv:13072196
- Seo D, Carmena JM, Rabaey JM, Maharbiz MM, Alon E. Model validation of untethered, ultrasonic neural dust motes for cortical recording. J Neurosci Methods. 2015;244:114–122. doi: 10.1016/j.jneumeth.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Seo D, et al. Wireless recording in the peripheral nervous system with ultrasonic neural dust. Neuron. 2016;91:529–539. doi: 10.1016/j.neuron.2016.06.034. [DOI] [PubMed] [Google Scholar]
- Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. 2014;5:1040. doi: 10.3389/fpsyg.2014.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanechi MM. Brain–machine interface control algorithms. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2017;25:1725–1734. doi: 10.1109/TNSRE.2016.2639501. [DOI] [PubMed] [Google Scholar]
- Shen R, Junhua L, Taya F, deSouza J, Thakor NV, Bezerianos A. Dynamic functional segregation and integration in human brain network during complex tasks. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2017;25:547–556. doi: 10.1109/TNSRE.2016.2597961. [DOI] [PubMed] [Google Scholar]
- Shenoy KV, Carmena JM. Combining decoder design and neural adaptation in brain–machine interfaces. Neuron. 2014;84:665–680. doi: 10.1016/j.neuron.2014.08.038. [DOI] [PubMed] [Google Scholar]
- Si Y, et al. Different decision-making responses occupy different brain networks for information processing: a study based on EEG and TMS. Cereb Cortex. 2018;29:4119–4129. doi: 10.1093/cercor/bhy294. [DOI] [PubMed] [Google Scholar]
- Silvani A, Calandra-Buonaura G, Dampney RA, Cortelli P. Brain–heart interactions: physiology and clinical implications. Philos Trans Ser A Math Phys Eng Sci. 2016;374:20150181. doi: 10.1098/rsta.2015.0181. [DOI] [PubMed] [Google Scholar]
- Sitaram R, et al. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci. 2017;18:86–100. doi: 10.1038/nrn.2016.164. [DOI] [PubMed] [Google Scholar]
- Talwar SK, Xu S, Hawley ES, Weiss SA, Moxon KA, Chapin JK. Rat navigation guided by remote control. Nature. 2002;417:37–38. doi: 10.1038/417037a. [DOI] [PubMed] [Google Scholar]
- Tang YP, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Taya F, Sun Y, Babiloni F, Thakor N, Bezerianos A. Brain enhancement through cognitive training: a new insight from brain connectome. Front Syst Neurosci. 2015;9:44. doi: 10.3389/fnsys.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault RT, Lifshitz M, Raz A. The self-regulating brain and neurofeedback: experimental science and clinical promise. Cortex. 2016;74:247–261. doi: 10.1016/j.cortex.2015.10.024. [DOI] [PubMed] [Google Scholar]
- Toppi J, et al. Investigating cooperative behavior in ecological settings: an EEG hyperscanning study. PLoS ONE. 2016;11:e0154236. doi: 10.1371/journal.pone.0154236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Berrio A, Nava-Mesa MO. The opioid system in stress-induced memory disorders: from basic mechanisms to clinical implications in post-traumatic stress disorder and Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:327–338. doi: 10.1016/j.pnpbp.2018.08.011. [DOI] [PubMed] [Google Scholar]
- Tost H, Champagne FA, Meyer-Lindenberg A. Environmental influence in the brain, human welfare and mental health. Nat Neurosci. 2015;18:1421–1431. doi: 10.1038/nn.4108. [DOI] [PubMed] [Google Scholar]
- Van der Wall E, Van Gilst W. Neurocardiology: close interaction between heart and brain. Netherlands Heart J. 2013;21:51–52. doi: 10.1007/s12471-012-0369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Erp J, Lotte F, Tangermann M. Brain–computer interfaces: beyond medical applications. Computer. 2012;45:26–34. [Google Scholar]
- Vecchiato G, et al. Spectral EEG frontal asymmetries correlate with the experienced pleasantness of TV commercial advertisements. Med Biol Eng Comput. 2011;49:579–583. doi: 10.1007/s11517-011-0747-x. [DOI] [PubMed] [Google Scholar]
- Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- Vidal JJ. Toward direct brain–computer communication. Annu Rev Biophys Bioeng. 1973;2:157–180. doi: 10.1146/annurev.bb.02.060173.001105. [DOI] [PubMed] [Google Scholar]
- Walker C, Brouwer BJ, Culham EG. Use of visual feedback in retraining balance following acute stroke. Phys Ther. 2000;80:886–895. [PubMed] [Google Scholar]
- Wang Z, et al. Effect of acupuncture in mild cognitive impairment and Alzheimer disease: a functional MRI study. PLoS ONE. 2012;7:e42730. doi: 10.1371/journal.pone.0042730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wu X, Wang Z, Ma Y. Implementation of a brain–computer interface on a lower-limb exoskeleton. IEEE Access. 2018;6:38524–38534. [Google Scholar]
- Wang X, Yan J, Wang Z, Li X, Yuan Y. Neuromodulation effects of ultrasound stimulation under different parameters on mouse motor cortex. IEEE Trans Biomed Eng. 2020;67:291–297. doi: 10.1109/TBME.2019.2912840. [DOI] [PubMed] [Google Scholar]
- Weng CB, Qian RB, Fu XM, Lin B, Han XP, Niu CS, Wang YH. Gray matter and white matter abnormalities in online game addiction. Eur J Radiol. 2013;82:1308–1312. doi: 10.1016/j.ejrad.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain–computer interface in humans. Proc Natl Acad Sci USA. 2004;101:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, McFarland DJ, Neat GW, Forneris CA. An EEG-based brain–computer interface for cursor control. Electroencephalogr Clin Neurophysiol. 1991;78:252–259. doi: 10.1016/0013-4694(91)90040-b. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, et al. Brain–computer interface technology: a review of the first international meeting. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2000;8:164–173. doi: 10.1109/tre.2000.847807. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain–computer interfaces for communication and control. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2002;113:767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- Wu D, Li CY, Yao DZ. Scale-free music of the brain. PLoS ONE. 2009;4:e5915. doi: 10.1371/journal.pone.0005915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Li C, Yao D. Scale-free brain quartet: artistic filtering of multi-channel brainwave music. PLoS ONE. 2013;8:e64046. doi: 10.1371/journal.pone.0064046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Kendrick KM, Levitin DJ, Li C, Yao D. Bach is the father of harmony: revealed by a 1/f fluctuation analysis across musical genres. PLoS ONE. 2015;10:e0142431. doi: 10.1371/journal.pone.0142431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, et al. Cyborg intelligence: recent progress and future directions. IEEE Intell Syst. 2016;31:44–50. [Google Scholar]
- Xiao J, Qu J, Li Y. An electrooculogram-based interaction method and its music-on-demand application in a virtual reality environment. IEEE Access. 2019;7:22059–22070. [Google Scholar]
- Xing Y, et al. Music exposure improves spatial cognition by enhancing the BDNF level of dorsal hippocampal subregions in the developing rats. Brain Res Bull. 2016;121:131–137. doi: 10.1016/j.brainresbull.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Xing Y, et al. Exposure to Mozart music reduces cognitive impairment in pilocarpine-induced status epilepticus rats. Cogn Neurodyn. 2016;10:23–30. doi: 10.1007/s11571-015-9361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, et al. Mozart, Mozart rhythm and retrograde Mozart effects: evidences from behaviours and neurobiology bases. Sci Rep. 2016;6:18744. doi: 10.1038/srep18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Qi H, Wan B, Yin T, Liu Z, Ming D. A hybrid BCI speller paradigm combining P300 potential and the SSVEP blocking feature. J Neural Eng. 2013;10:026001. doi: 10.1088/1741-2560/10/2/026001. [DOI] [PubMed] [Google Scholar]
- Yan T, Hui-Chan CW, Li LS. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke. 2005;36:80–85. doi: 10.1161/01.STR.0000149623.24906.63. [DOI] [PubMed] [Google Scholar]
- Yang M, et al. The effects of music intervention on functional connectivity strength of the brain in schizophrenia. Neural Plast. 2018;2018:2821832. doi: 10.1155/2018/2821832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao D (2017) Mesoscopic brainformatics. In: International conference on brain informatics, Springer, pp 315–324
- Yin E, Zhou Z, Jiang J, Yu Y, Hu D. A dynamically optimized SSVEP brain–computer interface (BCI) speller. IEEE Trans Biomed Eng. 2015;62:1447–1456. doi: 10.1109/TBME.2014.2320948. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Kim H, Filandrianos E, Taghados SJ, Park S. Non-invasive brain-to-brain interface (BBI): establishing functional links between two brains. PLoS ONE. 2013;8:e60410. doi: 10.1371/journal.pone.0060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Li Y, Long J, Gu Z. Surfing the internet with a BCI mouse. J Neural Eng. 2012;9:036012. doi: 10.1088/1741-2560/9/3/036012. [DOI] [PubMed] [Google Scholar]
- Yuan P, Chen X, Wang Y, Gao X, Gao S. Enhancing performances of SSVEP-based brain–computer interfaces via exploiting inter-subject information. J Neural Eng. 2015;12:046006. doi: 10.1088/1741-2560/12/4/046006. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: auditory–motor interactions in music perception and production. Nat Rev Neurosci. 2007;8:547–558. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]
- Zerafa R, Camilleri T, Falzon O, Camilleri KP. To train or not to train? A survey on training of feature extraction methods for SSVEP-based BCIs. J Neural Eng. 2018;15:051001. doi: 10.1088/1741-2552/aaca6e. [DOI] [PubMed] [Google Scholar]
- Zhang H, Guan C, Wang C. Asynchronous P300-based brain–computer interfaces: a computational approach with statistical models. IEEE Trans Biomed Eng. 2008;55:1754–1763. doi: 10.1109/tbme.2008.919128. [DOI] [PubMed] [Google Scholar]
- Zhang D, Maye A, Gao X, Hong B, Engel AK, Gao S. An independent brain–computer interface using covert non-spatial visual selective attention. J Neural Eng. 2010;7:16010. doi: 10.1088/1741-2560/7/1/016010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao Q, Jin J, Wang X, Cichocki A. A novel BCI based on ERP components sensitive to configural processing of human faces. J Neural Eng. 2012;9:026018. doi: 10.1088/1741-2560/9/2/026018. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu P, Guo D, Yao D. Prediction of SSVEP-based BCI performance by the resting-state EEG network. J Neural Eng. 2013;10:066017. doi: 10.1088/1741-2560/10/6/066017. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu P, Huang Y, Cheng K, Yao D. SSVEP response is related to functional brain network topology entrained by the flickering stimulus. PLoS ONE. 2013;8:e72654. doi: 10.1371/journal.pone.0072654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, et al. Efficient resting-state EEG network facilitates motor imagery performance. J Neural Eng. 2015;12:066024. doi: 10.1088/1741-2560/12/6/066024. [DOI] [PubMed] [Google Scholar]
- Zhang R, Li Y, Yan Y, Zhang H, Wu S, Yu T, Gu Z. Control of a wheelchair in an indoor environment based on a brain–computer interface and automated navigation. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2016;24:128–139. doi: 10.1109/TNSRE.2015.2439298. [DOI] [PubMed] [Google Scholar]
- Zhang T, et al. Structural and functional correlates of motor imagery BCI performance: insights from the patterns of fronto-parietal attention network. NeuroImage. 2016;134:475–485. doi: 10.1016/j.neuroimage.2016.04.030. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Jin J, Wang X. Sparse Bayesian learning for obtaining sparsity of EEG frequency bands based feature vectors in motor imagery classification. Int J Neural Syst. 2017;27:1650032. doi: 10.1142/S0129065716500325. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Zhou G, Jin J, Wang B, Wang X, Cichocki A. Multi-kernel extreme learning machine for EEG classification in brain–computer interfaces. Expert Syst Appl. 2018;96:302–310. [Google Scholar]
- Zhang Y, et al. Two-stage frequency recognition method based on correlated component analysis for SSVEP-based BCI. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2018;26:1314–1323. doi: 10.1109/TNSRE.2018.2848222. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yuan S, Huang L, Zheng X, Wu Z, Xu K, Pan G. Human mind control of rat cyborg’s continuous locomotion with wireless brain-to-brain interface. Sci Rep. 2019;9:1321. doi: 10.1038/s41598-018-36885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller TR, Dowling AL, Herzig CT, Iannacone EA, Gauger KJ, Bansal R. Thyroid hormone, brain development, and the environment. Environ Health Perspect. 2002;110(Suppl 3):355–361. doi: 10.1289/ehp.02110s3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner C, Belardinelli P, Muller-Dahlhaus F, Ziemann U. Closed-loop neuroscience and non-invasive brain stimulation: a tale of two loops. Front Cell Neurosci. 2016;10:92. doi: 10.3389/fncel.2016.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]