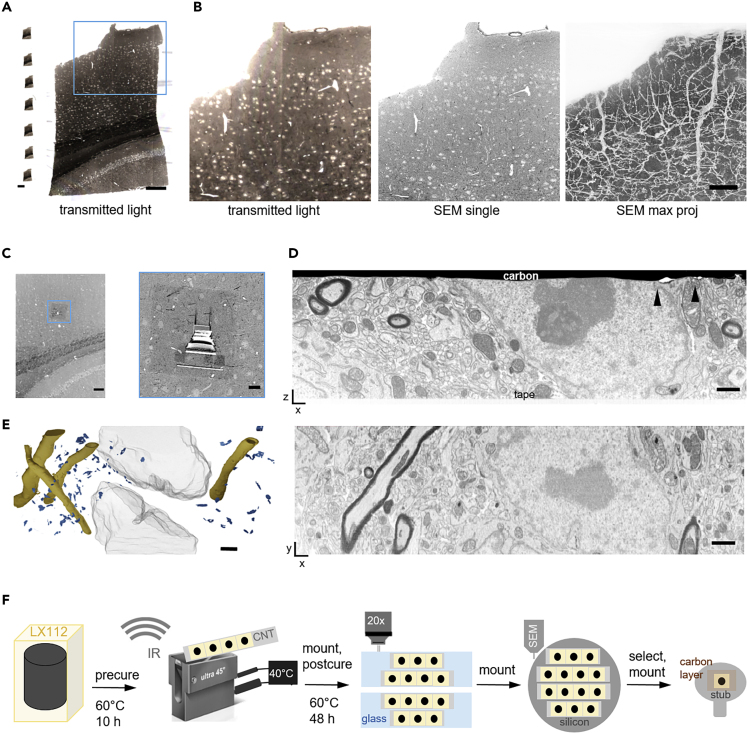
Figure 4.
Serial Semithick Sectioning for Targeted FIB-SEM.
Serial 5-μm-thick sections of mouse cortex were collected on CNT tape.
(A) Overview of several consecutive sections on tape (left) and a single transmitted light micrograph (20x objective, right). Scale bars, 2 mm and 100 μm, respectively.
(B) Transmitted light (left) and BSD images (10 × 10 × 5,000 nm resolution) of a selected region on a single section shown in (A) and maximum projection of all 93 sections revealing the vasculature morphology (right). Scale bar, 20 μm.
(C) A random cortical FIB target site was chosen (blue box) (left). The selected region was prepared for FIB-SEM by carbon deposition and trench milling (right). Scale bars, 100 and 10 μm, respectively.
(D) Cross section after FIB-SEM preparation showing the section—CNT tape adhesion site and the surface covered by a carbon layer (top). Defects in the surface layer topology are highlighted (black arrowheads). After the FIB-SEM run an xy section was reconstructed (bottom) from 2000 SE images (resolution 5 × 5 × 5 nm3, bottom). Scale bar, 1 μm.
(E) 3D model of the FIB-SEM stack revealing myelinated axons (yellow), post synaptic densities (blue), and neuronal nuclei (gray). Scale bar, 2 μm.
(F) Schematic of the ATUM-FIB strategy: sequential resin (LX112) curing and heated ultramicrotomy are required for semithick sectioning. Serial sections are attached onto glass slides for light microscopy and remounted onto silicon wafers for serial SEM imaging and target selection. A section of interest is remounted, adhered onto a standard sample holder, and carbon-coated for FIB-SEM examination.
See Figure S2 for photographs of the different processing steps.