Summary
Proper immune system function hinders cancer development, but little is known about whether genetic variants linked to cancer risk alter immune cells. Here, we report 57 cancer risk loci associated with differences in immune and/or stromal cell contents in the corresponding tissue. Predicted target genes show expression and regulatory associations with immune features. Polygenic risk scores also reveal associations with immune and/or stromal cell contents, and breast cancer scores show consistent results in normal and tumor tissue. SH2B3 links peripheral alterations of several immune cell types to the risk of this malignancy. Pleiotropic SH2B3 variants are associated with breast cancer risk in BRCA1/2 mutation carriers. A retrospective case-cohort study indicates a positive association between blood counts of basophils, leukocytes, and monocytes and age at breast cancer diagnosis. These findings broaden our knowledge of the role of the immune system in cancer and highlight promising prevention strategies for individuals at high risk.
Subject Areas: Immunology, Cancer Systems Biology, Cancer
Graphical Abstract
Highlights
-
•
Cancer risk genetic variants linked to immune/stromal cell tissue content
-
•
SH2B3 associated with BRCA1/2 cancer risk and immune cell counts
-
•
Peripheral immune cell types linked to breast cancer age at diagnosis
Immunology; Cancer Systems Biology; Cancer
Introduction
The immune system maintains organismal integrity and function by continuously protecting itself from exogenous and endogenous assaults. The concept of “immunological surveillance of cancer” was first proposed by Burnet in 1970 (Burnet, 1970) and developed by Thomas about a decade later (Thomas, 1982). In this theory, the immune system inactivates or eliminates cancer-prone cells that are detected early in normal tissue (Ribatti, 2017). Although this idea remains a matter of debate, it is clear that some immune factors decisively influence cancer development and progression. Immunosuppression due to primary immunodeficiency or due to therapies administered to prevent organ transplant rejection and certain virus infections are associated with an increased risk of some cancers (Mortaz et al., 2016). In parallel, studies of mouse models with defined genetic alterations have demonstrated the relevance of immunosurveillance; for example, loss of Nkg2d, which encodes the activating receptor of natural killer (NK) and T cells, increases the risk of spontaneous neoplasms (Guerra et al., 2008).
Results from genome-wide association studies (GWASs) have identified risk-associated variants in loci coding for immune regulatory factors, such as NKG2D for cervical cancer risk (Chen et al., 2013). Indeed, pathway-based analyses of GWAS results have highlighted the involvement of immune-related processes in susceptibility to certain cancer types (Michailidou et al., 2017). In parallel, many germline genetic variants can influence immune cell infiltration in tumors (Lim et al., 2018). Therefore, immune-centered investigations of normal or precancerous tissue could yield fundamental evidence for improving cancer risk estimation and prevention (Spira et al., 2017). However, whether common genetic variants linked to cancer risk alter immune cell contents in the corresponding cancer target tissue, and/or at the systemic level, remains largely undetermined.
The balance between immunological surveillance and tolerance is determined from a complex interplay between different types of immune cells and other classes of stromal cells (Vinay et al., 2015; Gonzalez et al., 2018). Here, we describe an integrative analysis of genetic and transcriptome data from tissue defined as normal and located adjacent to surgically removed tumors, and from primary tumors analyzed by The Cancer Genome Atlas (TCGA) (Cancer Genome Atlas Research Network et al., 2013). This enables us to identify immune and stromal (hereafter “immune/stromal”) cell tissue content associations with the risk of several human cancer types. Beyond single variants, polygenic risk scores (PRSs) also show associations with differences in inferred immune/stromal cell tissue contents. Consistent associations among immune cell signatures, PRSs, and age at diagnosis suggest that higher immune cell infiltration reduces the risk of breast cancer. We identify the lymphocyte SH2B adaptor 3 (LNK/SH2B3) locus as linking immune cell counts and breast cancer risk, including that from BRCA1/2 mutation carriers. To evaluate this connection further, we assess associations between breast cancer age at diagnosis and immune cell counts measured at diagnosis in routine clinical blood tests; the results further suggest that peripheral immune cell status influences breast cancer risk. Collectively, these findings may broaden our current knowledge of the biological basis of cancer risk and thereby suggest strategies for cancer prevention.
Results
Strategy to Evaluate Immune/Stromal Cell Tissue Contents that Influence Cancer Risk
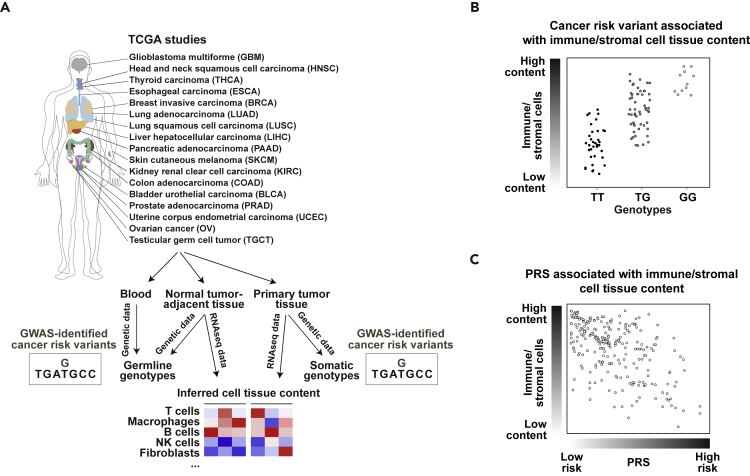
TCGA has greatly increased our knowledge of human cancer through multilayer biological analyses, which include genetic and gene expression profiling of tissue considered to be normal and situated adjacent to the cancer (hereafter referred to as “normal”) and primary tumors (Liu et al., 2018). In parallel, many successful GWASs have identified hundreds of germline genetic variants associated with the risk of common cancer types (Torkamani et al., 2018). By compiling GWAS results, we assigned cancer risk variants to 17 TCGA projects based on tissue of origin correspondences (Figure 1A lists the cancer study acronyms, and Table S1 lists the cancer risk variants). As deconvolution analyses of bulk gene expression enable robust inference of cell type content in heterogeneous samples (Avila Cobos et al., 2018), deduced cell content in normal tissue and tumors can be assessed for associations with cancer risk variants in multivariate analyses (Figure 1B). Moreover, as differences in cancer risk are more accurately defined by combinations of key variants in PRSs (Torkamani et al., 2018), it might be possible to better define the relevance of the immune/stromal cells by analyzing associations between PRSs and their corresponding signatures (Figure 1C; Table S2 summarizes the number of normal tissue and primary tumor samples available for each subsequent analysis).
Figure 1.
Strategy for Assessing the Effect of Immune/Stromal Cell Tissue Content on Cancer Risk
(A) TCGA cancer projects analyzed in this study and data analysis workflow.
(B) Association between gene expression-inferred immune/stromal cell tissue content and GWAS-identified risk variant.
(C) Association between gene expression-inferred immune/stromal cell tissue content and cancer PRS.
To infer immune/stromal cell contents in normal and primary tumor samples using bulk tissue RNA sequencing (RNA-seq) data from TCGA, we applied a consensus-signature approach benchmarked against other methods (ConsensusTME; Jiménez-Sánchez et al., 2019). Using this approach, the computed immune/stromal estimations in the 17 identified TCGA datasets were typically found to be positively correlated with two other methods (Figure S1), as well as with estimates of leukocyte content measured by a different method (Taylor et al., 2018) (Figure S2). In turn, the estimates were generally found to be negatively correlated with aneuploidy (Figure S3), as expected (Taylor et al., 2018). In addition, the immune/stromal cell TCGA estimates showed significant differences between primary tumors with low or high levels of CD274/PDL1 and CD279/PDCD1 expression (Figures S4 and S5). Applying the method to RNA-seq data from whole blood samples of healthy adults also revealed positive correlations with immune cell enumerations using fluorescence-activated cell sorting (Newman et al., 2019) (Figure S6). Moreover, the estimates from this method were found to be highly correlated (Spearman's ρ > 0.75) with the numbers of immune/stromal cells used to generate 100 pseudo-bulk breast tumors (Figure S7 and Methods).
To further assess the validity of the inferred immune cell contents in TCGA, the deduced scores for each setting were assessed for associations with defined immune benchmark genes (Methods). In most settings, each inferred immune cell type content was found to be positively correlated with the expression of the assigned benchmark; the average Pearson's correlation coefficient values for all signature-benchmark pairs were 0.52 and 0.60 in the normal tissue and primary tumor sets, respectively (Figure S8 and Table S3). To assess further the coherence of the inferred immune cell contents, the corresponding scores were tested for association with the activity status of immune-related signaling pathways (Cubuk et al., 2018). This analysis revealed coherent clustering of immune and stromal cell types in normal tissue (Figure S9).
Identification of Cancer Risk Variants Linked to Differences in Immune/Stromal Cell Tissue Content
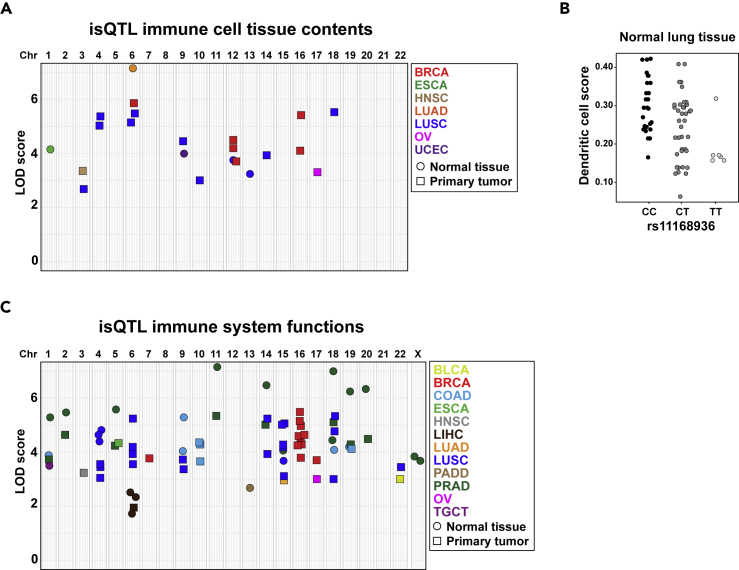
A total of 1,453 cancer risk variants were compiled from various sources; 214 of these were directly genotyped in TCGA, and the rest were imputed. After applying quality controls and filtering criteria (Methods), 627 and 966 variants were analyzed as potential immune/stromal quantitative trait loci (isQTLs). The isQTLs were identified using multivariate regressions including covariates of gender (when informative), age at diagnosis, tumor stage, and histology. The significance of the associations in each setting was concluded from 1,000 permutations (Methods). Tumor data were also analyzed because germline risk alleles are frequently associated with defined cancer histopathological and biomarker features (Michailidou et al., 2017). To avoid redundant tests, only cell signatures with eigenvalues >1 were examined in each setting (Table S4). Through this methodology, 22 significant isQTLs were identified. These comprised normal tissue corresponding to esophageal carcinoma (ESCA), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), and uterine corpus endometrial carcinoma (UCEC), and primary tumors of breast cancer (BRCA), head and neck squamous cell carcinoma (HNSC), LUSC, and ovarian serous cystadenocarcinoma (OV) (Figure 2A and Table S5).
Figure 2.
isQTLs Linked to Cancer Risk
(A) Graph indicating the association values (LOD scores) and relative chromosome locations of 22 isQTLs identified in normal tissue and primary tumor analyses (Table S5).
(B) isQTL rs11168936 for dendritic cell content in normal tissue corresponding to LUSC.
(C) Graph showing the LOD scores and relative chromosome locations of isQTLs identified using five TCGA-defined immune signatures (Table S6).
Several of the identified isQTLs involved differences in endothelial and fibroblast cell content (Table S5), and these signals may also indicate links with immune cell differences: for instance, rs4072037 is associated with endothelial cell content in normal esophageal tissue, and this variant corresponds to a cis-expression (cis-e) QTL in several normal tissue types (GTEx Consortium, 2013) of genes whose products are functionally relevant in the immune system and biology of endothelial cells (Stenina-Adognravi, 2014), including GBA, GBAP1, TSP3/THSB3, and MUC1, which are locus-mapped genes. In addition, the cancer pleiotropy rs11168936 variant (Fehringer et al., 2016) is associated with differences in fibroblast content in normal lung tissue corresponding to LUSC, and this variant is a cis-eQTL for C1QL4 in several normal tissue types (GTEx Consortium, 2013). Intriguingly, C1q is a regulator of dendritic cell maturation (van Kooten et al., 2008), and we found this variant also to be associated with dendritic cell content (Figure 2B). In normal lung tissue corresponding to LUAD, rs17078110 is associated with B cells, and this locus codes for SASH1, a regulator of TLR4 signaling and cytokine production (Dauphinee et al., 2013). Among the isQTLs identified in tumors, rs3764419 is associated with cytotoxic cell content in OV. This variant is a cis-eQTL for ATAD5 (GTEx Consortium, 2013), whose product is essential for proper B cell biology and immunoglobulin production (Zanotti et al., 2015). Overall, these data suggest that some cancer risk variants are associated with immune/stromal cell tissue content, and that this link is mediated by alterations in genes of functional importance to the immune system.
Identification of Cancer Risk Variants Associated with Immune System Functions in Target Tissue and Primary Tumors
The TCGA consortium examined 160 immune system-related gene expression signatures across hundreds of tumors and identified five of them as being informative for cancer classification: IFN-γ response, lymphocyte infiltration score, macrophage regulation, TGF-β response, and wound healing (Thorsson et al., 2018). Therefore, we sought to expand on the aforementioned cell-type-based associations by analyzing these five additional signatures using the same method as introduced earlier: multivariate regression with significance determined from 1,000 permutations. This study identified 75 isQTLs, of which 11 variants had been identified in the previous isQTL analyses, which represents a significant concordance (Fisher's exact test, p < 0.0001; Figure 2C and Table S6). Taking both analyses into account suggests that the risk of 13 cancer types may be influenced by immune/stromal cell tissue content.
Of the 57 unique variants identified from all isQTLs, five were linked to tumor suppressor genes with recognized roles in the immune system: CDKN2A/B, DCC, MUC1, and SASH1. In addition, genomic enhancers identified in T helper, regulatory, effector, memory, and mononuclear cells were significantly over-represented in this unique variant set relative to all human variants: > 2-fold enrichments, binomial test p values <0.05 (Ward and Kellis, 2012). Consistent with this observation, 8 (14%) variants corresponded to expression (e) QTLs from 18 immune-related genes in normal human tissue (GTEx Consortium, 2013) and 13 (25%) corresponded to eQTLs identified in CD4+ and/or CD8+ T cells (Kasela et al., 2017) (Tables S5 and S6). To evaluate the relevance of these observed percentages, we examined the expected proportions when considering all cancer risk variants studied; lower percentages were identified in both analyses, with expected proportions of 11% (115/1,079) for eQTLs of immune-related genes in normal human tissue (GTEx Consortium, 2013) and 14% (151/1,079) for eQTLs in CD4/8+ T cells (Kasela et al., 2017). We then examined whether the eQTL gene targets documented within the isQTLs were functionally coherent by determining the proportion of significant gene expression-immune/stromal cell signature correlations and comparing the results with those from equivalent 1,000 random gene sets. Both isQTL sets (Tables S5 and S6) included a higher proportion of eQTL gene targets that were positively correlated with immune/stromal cell signatures than expected by chance (Figure S10). Finally, variants correlated (r2 > 0.8) with each isQTL were intersected with various functional genomic data from B cells, monocytes, and CD4+ and CD8+ T cells, and for potential effects on protein coding sequences (Methods). These analyses identified two additional candidate genes (LIF and OSM) with established functions in the immune system, being involved in cytokine signaling (Table S7). Together, these data indicate that a substantial proportion of the isQTLs identified influence genes whose expression is associated with immune system functions.
PRS Associations with Immune/Stromal Cell Tissue Content Highlight Breast Cancer Risk
The effects of individual cancer risk variants are generally small, but their combinations within PRSs can potentially identify individuals who are at substantially higher risk than average for the population (Torkamani et al., 2018). Therefore, reported PRSs were computed in the corresponding normal tissue and primary tumor TCGA settings and evaluated for associations with immune/stromal cell contents using multivariate analyses as described earlier. The study of normal tissue was limited to breast. Despite the valuable TCGA resource, the available sample size sets limited the detection of nominal significant associations to those with correlation coefficients of r > 0.3 in normal breast and of r > 0.12 in BRCA; higher correlations would be required for all other normal or tumor settings (Figure S11).
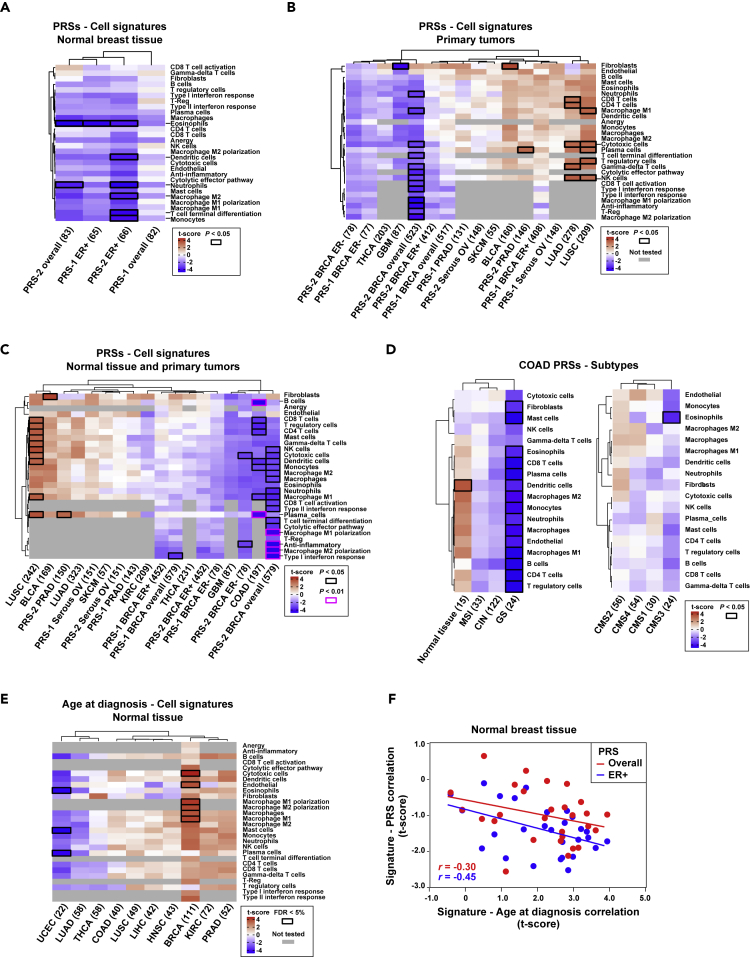
In normal breast, most immune/stromal cell contents tended to be negatively correlated with the corresponding PRSs; the PRS cell signature correlation coefficients for overall and estrogen receptor (ER)-positive breast cancer were significantly less than zero (p values <0.001; Figure 3A). The ER-negative PRS could not be computed because of the relatively low number of normal samples of this subtype and with complete data. Analogous limitations were encountered when attempting to analyze triple-negative breast cancer (TNBC) and human epidermal growth factor receptor 2 (HER2)-positive breast cancers, and there were no HER2-specific PRSs to analyze whatsoever. Potentially protective cell types (i.e., those exhibiting a nominally significant negative correlation between cell content and PRS) in the aforementioned two breast cancer settings included dendritic cells, eosinophils, macrophage M2, monocytes, neutrophils, and T cell terminal differentiation (Figure 3A).
Figure 3.
Associations between Immune/Stromal Cell Signatures and PRSs
(A) Unsupervised clustering of the results of the regression analysis between cell signatures and PRSs in normal breast tissue. The y axis depicts the cell type signatures, and the x axis shows the PRSs. Sources #1 and #2 of the PRSs are detailed in Methods. ER+ and ER− indicate estrogen receptor-positive and estrogen receptor-negative subsets, respectively. The maximum sample size used in each analysis is shown. The color scale (t-score) is calculated as the β estimate divided by the standard error. Nominally significant associations are indicated by black-outlined rectangles.
(B) Unsupervised clustering of the coefficients of the regression of cell signature values in primary tumor TCGA studies and the corresponding PRSs. The gray-filled rectangles indicate “not tested” correlations because the corresponding cell signatures were only defined for breast cancer.
(C) Unsupervised clustering of the coefficients of the regression of cell signature values in combined normal tissue and primary tumor datasets, and the corresponding PRSs. The regression p values <0.01 are also indicated as depicted in the inset.
(D) Unsupervised clustering of the coefficients of the regression analysis between PRSs and cell signatures in combined normal tissue and primary tumors of the COAD study, divided by cancer subtypes.
(E) Unsupervised clustering of the results of the regression analysis of cell signatures in normal tissue and age at diagnosis across TCGA studies. Associations significant at a false discovery rate (FDR) < 5% are indicated by black-outlined rectangles.
(F) Negative correlations between the coefficients of regressions of immune/stromal cell contents and age at diagnosis or PRSs in normal breast tissue, for all cases and only ER-positive cases. The correlation coefficients are shown.
In addition to the breast cancer PRSs, eight other scores (Fritsche et al., 2018) were examined in their corresponding primary tumor TCGA settings. The distribution of the correlation coefficients between immune/stromal cell tissue content and the PRS was again found to be less than zero not only in BRCA but also in in glioblastoma multiforme (GBM; with a major contribution for fibroblast content) and thyroid carcinoma (THCA; Figure 3B). Conversely, positive correlations were detected in bladder urothelial carcinoma (BLCA), OV, prostate adenocarcinoma (PRAD), skin cutaneous melanoma (SKCM), and, principally, in LUAD and LUSC (Figure 3B). Conversely, positive correlations were detected in BLCA, serous OV, PRAD, SKCM, and, principally, in LUAD and LUSC (Figure 3B). Therefore, risk stratification based on PRSs may also be linked to differences in immune/stromal cell content in normal and/or tumor tissue. LUAD and LUSC PRSs shared positive correlations (p < 0.05) with cytotoxic and NK cell tissue contents; however, these associations may be influenced by smoking status, because LUAD current smokers showed an opposite trend (Figure S12).
Combined analyses of normal tissue and primary tumor data further suggested common protective effects for high immune cell content in breast and colorectal tissue, and also potentially in brain and a few other settings (Figure 3C). In contrast, high immune cell content might principally increase the risk of lung, bladder, and pancreatic cancer (Figure 3C), although, as already noted, smoking may influence these associations. Then, analyses of COAD subtypes (Methods) suggested protective effects for high immune cell content in genomic stable tumors (Figure 3D, left panel), but this association might be biased due to PRS development in overall incident cases. When analyzing the COAD molecular subtypes, lower risk of CSM3 might also be associated with higher immune cell content (Figure 3D, right panel). The sample sets of these subtype analyses were relatively small to obtain robust conclusions, but, when compared with normal colorectal tissue, an opposite trend was observed (Figure 3D, left panel), which suggests that immune cell infiltration has different roles between normal tissue and tumors.
As described earlier, the normal breast and BRCA settings both showed PRS-cell signature negative correlations. To assess these observations further, the correlation estimates were compared with those from similar analyses using age at diagnosis instead of the PRSs. In normal breast tissue, the immune/stromal cell contents tended to show positive correlations with age at diagnosis (p < 0.001; Figure 3E). Consequently, negative correlations were detected between the estimates from the two parallel analyses, considering all cases or solely ER-positive cases (Figure 3F). Therefore, relatively higher immune/stromal cell content in normal breast might be a factor protecting against development of malignancy.
SH2B3 Connects Immune Cell Tissue Content with Breast Cancer Risk
The identified cancer risk isQTLs could be explained by peripheral alterations in immune cells. Examination of GWAS results for blood cell traits revealed that the tumor COAD isQTL rs12412391 in chromosome 10 (Table S6) is in linkage disequilibrium (r2 = 0.93) with rs11190133, which is associated with differences in platelets in the UK Biobank study (Astle et al., 2016). These variants constitute an eQTL of NKX2-3 (GTEx Consortium, 2013), and, remarkably, loss of the mouse ortholog causes developmental alterations in the spleen, colonic crypts, and lymphocyte tissue homing (Pabst et al., 1999). In addition to this locus, the tumor BRCA isQTL rs11065979 in chromosome 12 (Table S5) was associated with blood count differences in basophils, erythrocytes, eosinophils, leukocytes, monocytes, and neutrophils in the UK Biobank study (Astle et al., 2016) (Table S8). The same study also indicated an association with breast cancer risk (p = 0.0003; Table S8). This variant has also been linked to cancer pleiotropy (Fehringer et al., 2016) and psoriasis (Tsoi et al., 2017), among other traits (GWAS Catalog). A variant in linkage disequilibrium, rs3184504 (r2 = 0.89), had also been associated with breast cancer risk (Fehringer et al., 2016), serum IgA levels (Jonsson et al., 2017), and various autoimmune diseases (Webb and Hirschfield, 2016), among other traits (GWAS Catalog).
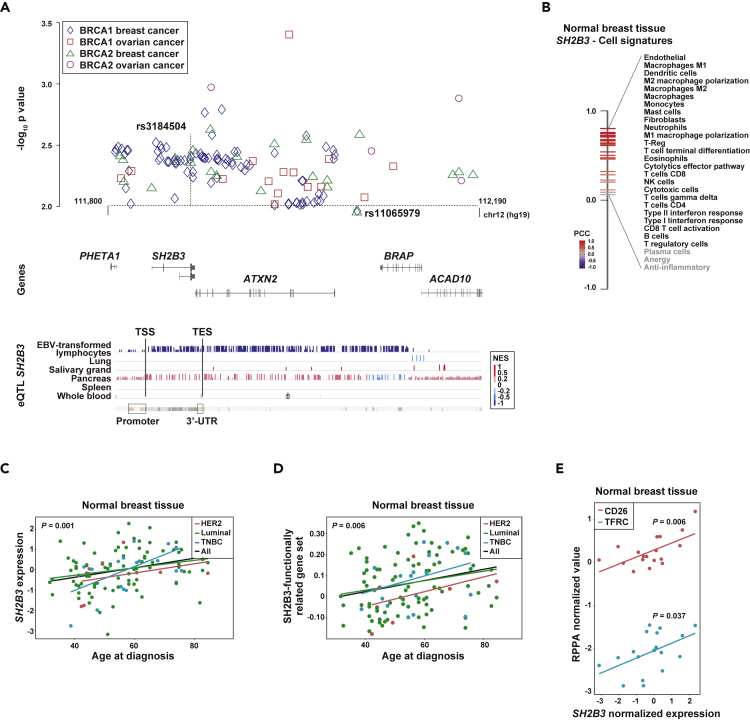
To investigate further the role of the isQTL identified in chromosome 12 and linked to breast cancer risk, we analyzed association results from BRCA1/2 mutation carriers. Both depicted variants showed nominal associations with breast cancer risk in women carriers of germline BRCA1 or BRCA2 mutations: BRCA1 mutation carriers, rs11065979 hazard ratio (HR) = 0.96, 95% confidence interval (CI) 0.92–0.99, p = 0.018; rs3184504 HR = 0.95, 95% CI 0.92–0.99, p = 0.006; BRCA2 mutation carriers, rs11065979 HR = 0.94, 95% CI 0.90–0.99, p = 0.019; and rs3184504 HR = 0.93, 95% CI 0.89–0.98, p = 0.003. Then, wider examination of this region in chromosome 12 identified several genetic associations (p < 0.01) with breast and/or ovarian cancer risk in these women (Figure 4A and Table S9).
Figure 4.
The SH2B3 Locus Shows Associations with Breast Cancer Risk and Immune Cell Features
(A) Graph showing the chromosome 12 association results (-log10 p value, y axis) with breast and ovarian cancer risk (as depicted in the inset) in women carriers of BRCA1/2 mutations. The rs3184504 and rs11065979 variants are indicated.
(B) Rank of expression correlations (Pearson's correlation coefficients [PCCs]) between SH2B3 and immune/cell signatures in normal breast. All PCCs were >0, but three of them did not reach nominal significance (marked gray).
(C) Positive correlation between SH2B3 expression in normal breast and age of diagnosis of breast cancer. The trend lines for all cases and subtypes (luminal, HER2-positive, and triple-negative breast cancer [TNBC]) are shown. The correlation p value from the multivariate regression analysis is shown.
(D) Positive correlation between SH2B3 functionally related gene set in normal breast and age of diagnosis of breast cancer.
(E) Positive correlation between SH2B3 expression and CD26 and TFRC protein expression as measured by TCGA reverse-phase protein array (RPPA) assays. The correlation p value from the multivariate regression analysis is shown.
The chromosome 12 locus identified here includes many eQTL signals for SH2B3 in EBV-transformed lymphocytes and normal tissue (Figure 4A, bottom panel). Next, to evaluate potential causality linked to SH2B3, complementary gene expression analyses were performed using the normal breast tissue TCGA data. First, the expression of SH2B3 was found to be positively correlated with most of the immune cell/stromal cell signatures (Figure 4B); second, SH2B3 expression was also found to be positively correlated with age at diagnosis, adjusted for tumor stage and regardless of cancer subtype (Figure 4C); third, an 84-gene signature corresponding to gene and protein functional relationships with mouse Sh2b3 and/or human SH2B3 (Huan et al., 2015) was also positively correlated with age at diagnosis (Figure 4D); and last, SH2B3 expression was positively correlated with the protein measures of CD26, cell surface glycoprotein receptor important for T cell activation (Klemann et al., 2016), and TFCR, transferrin receptor required for erythropoiesis and immune system development (Jabara et al., 2016) (Figure 4E). In addition, the association between SH2B3 expression in normal breast and age at diagnosis was replicated in an independent dataset (Terunuma et al., 2014): n = 47, r = 0.30, p = 0.039. Therefore, an identified isQTL may influence breast cancer risk through perturbation of SH2B3 expression, which is expected to be fundamental for accurate systemic development and function of immune cell populations (Li et al., 2000; Velazquez et al., 2002; Jabara et al., 2016).
Peripheral Immune Cell Counts Are Associated with Breast Cancer Risk
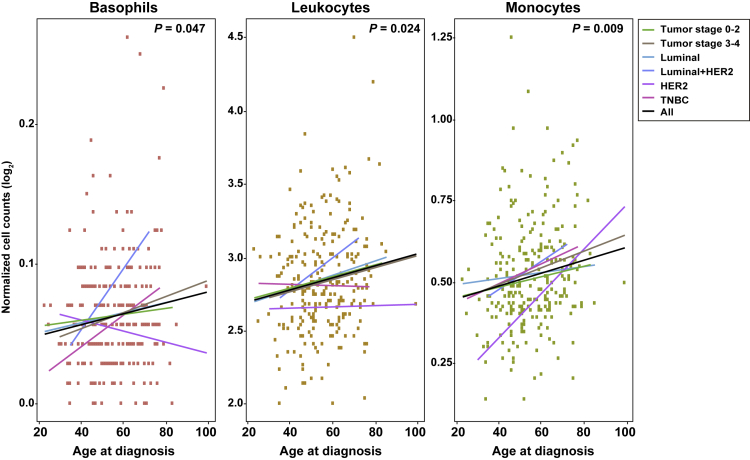
To assess the proposed link between breast cancer risk and peripheral immune cell counts, which in turn might be influenced by specific genetic variants and gene candidates, a retrospective case-cohort study was performed. Data on age at diagnosis, tumor stage and subtype, and blood test results from 259 breast cancer cases were compiled in a tertiary referral hospital (Methods). The cases were randomly selected from clinical health records and showed an average age at diagnosis of 55.6 years, 95% CI 54.0–57.1 years. The blood test data were those collected on the date closest to diagnosis: 6 patients had the blood test on the same date as their diagnosis, 40 were earlier (on average 40 days before), and 182 were later (on average 45 days later): the average time between the blood test and disease diagnosis was 23.9 days, 95% CI 16.3–31.5 days. A multivariate regression analysis including tumor stage and subtype revealed three immune cell types to be significantly (p < 0.05) positively correlated with age at diagnosis: basophils, leukocytes, and monocytes (Figure 5). The trends were consistent for different tumor stages (0–2 and 3–4) and the major cancer subtype (i.e., luminal); the smaller patient sets of HER2-postive breast cancer (n = 18) and TNBC (n = 17) showed greater variability (Figure 5). The neutrophil to lymphocyte ratio, which is an established rate associated with breast cancer prognosis (Ethier et al., 2017), was not found to be associated with age at diagnosis in this study (p = 0.65).
Figure 5.
Positive Correlation between Peripheral Immune Cell Counts around Time of Diagnosis and Breast Cancer Age at Diagnosis
Positive correlations between basophil, leukocyte, and monocyte blood counts and age of diagnosis of breast cancer in a retrospective case-cohort hospital-based study. The value of p shown here is that associated with the coefficient calculated as part of the multivariate regression analysis. The trends for tumor subgroups are shown as depicted in the inset.
Discussion
The results of this study support the idea that the risk of certain cancers is influenced by the content of immune/stromal cells in the target tissue and/or by differences in peripheral immune cell counts. Of the 17 cancer settings analyzed, 57 risk loci comprising 13 cancer types were associated with differences in immune/stromal cell content with respect to the corresponding normal tissue and/or primary tumors. The gene candidates linked to these associations include several with key functions in the immune system, and they show significant enrichments in immune-related regulatory features and expression profiles. Detection of associations between immune/stromal cell signatures and PRSs provide further evidence that differences in these cell contents influence cancer risk. Nevertheless, the role of some cell types is multifaceted; for example, endothelial cells can regulate trafficking and activation of immune cells in a given tissue, but, critically, also determine angiogenesis (Hendry et al., 2016). Similarly, a given genetic variant may influence the expression of more than one gene target and/or indirectly alter the immune system by different mechanisms, such as by provoking oncogenic-induced signals.
Unexpectedly, there appear to be opposing cancer risk effects for immune cell contents across cancer types. These might be due to differences in tissue microenvironment conditions, such as inflammation caused by smoking or other factors (Shalapour and Karin, 2015). However, the study was limited by the relatively low numbers of normal tissue samples available for analysis, and, potentially, by gene expression alterations in normal tissue adjacent to neoplasms. This study had more power to detect significant results in normal breast tissue and BRCA and, consequently, the results prove to be more relevant and coherent in these settings. Carrying out similar analyses in other normal and cancer tissue contexts would appear to be worthwhile. Such additional studies would benefit from complementary molecular marker and signaling analyses, which would definitively establish the functional consequences of inferred cell alterations.
At the same time as providing insight into the biological basis of cancer initiation, this study yields data that could be useful for analyses of cancer risk and prevention. Associations of PRSs with immune cell signatures could inform preventive strategies by modulating specific cell functions and/or their signaling molecules in individuals at high risk (Spira et al., 2017). This idea is particularly relevant in breast cancer. Our study shows consistent associations between immune/stromal cell signatures and breast cancer PRSs or age at diagnosis in normal tissue. A recognized risk locus connects differences in most peripheral immune cell types to breast cancer risk. This locus harbors the SH2B3 gene, which is altered in hematological neoplasms and autoimmune diseases (Maslah et al., 2017, p. 3). Common genetic variation at this locus has been linked to cancer pleiotropy, including breast cancer susceptibility (Hung et al., 2015; Fehringer et al., 2016). We extend these observations by identifying potential associations with breast and ovarian cancer risk in BRCA1/2 mutation carriers. Our study shows consistent expression correlations of SH2B3 or SH2B3 functionally related genes with age at diagnosis using normal breast tissue data. Thus, pharmacological enhancement of SH2B3 function might reduce cancer risk in individuals with high PRSs and/or carriers of BRCA1/2 mutations. However, the functional impact on SH2B3 remains to be established, and, therefore, prospective studies determining the expression and/or functional differences of SH2B3 among individuals with specific alleles in the corresponding locus, and their associations with peripheral immune cell counts and cancer risk, are needed.
The effect of systemic differences of immune cell counts on breast cancer risk is further supported by unexpected associations between basophil, leukocyte, and monocyte blood counts and age at diagnosis from a retrospective case-cohort study. Relatively low monocyte counts collected over a 1-year period of disease diagnosis have recently been associated with increased breast cancer risk (Kresovich et al., 2020). However, high baseline leukocyte counts in a prospective study of postmenopausal women were found to be associated with increased breast cancer incidence (Margolis et al., 2007). In our study, we aimed to assess whether individuals' status of having relatively low peripheral immune cell counts was associated with initial cancer development, hypothetically due to reduced immunosurveillance. Our results are consistent with this explanation, and among other factors, altered SH2B3 function might give rise to these observations. As a whole, the results of this study may be useful for improving cancer risk estimation, and for identifying preventive approaches.
Limitations of the Study
The present report identifies cancer-associated genetic variants and polygenic risk scores linked to the alteration of immune and/or stromal cell systemic and/or tissue contents. These links could explain the greater cancer risk. However, the study has several limitations that should be borne in mind. The cell content inferences were based on gene expression profiles, and therefore, molecular and cellular analyses are required to corroborate them and accurately assess their functional consequences. The observed associations could also be indirect in some instances. The study was also limited by the original sample sets, and observed associations could be confounded by other factors, such as the level of tissue inflammation, individual hormonal status, and lifestyle aspects. The genetic basis of the proposed associations between blood cell count and age at breast cancer diagnosis in the studied cohort remains unknown, and it is unclear whether similar associations exist in BRCA1/2 mutation carriers.
Resource Availability
Lead Contact
Further information and requests for resources and data should be directed to and fulfilled by the Lead Contact Miquel Angel Pujana (mapujana@iconcologia.net).
Materials Availability
No materials were generated.
Data and Code Availability
TCGA data were obtained from the Genomic Data Commons Data Portal (https://portal.gdc.cancer.gov) and from the corresponding consortium publications. Individual genetic data were obtained following specific approval: dbGaP Data Access Committee project #11689. The R software algorithms developed by others and applied in this study are detailed in the Transparent Methods supplemental file. A complete pipeline was implemented and is available at https://github.com/pujana-lab/systematicQTL/.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The results presented here are partly based on data generated by the TCGA Research Network (https://www.cancer.gov/tcga), and we would like to express our gratitude to the TCGA consortia and their coordinators for providing the data and clinical information. This study was supported by the following patient foundations in Catalonia: DACMA, GINKGO, Sosciathlon, and “Viladecans Contra el Cáncer”. The work was also supported by grants from the Carlos III Institute of Health funded by FEDER funds – a way to build Europe – (ISCIII; Ministry of Science, Innovation and Universities; PI16/00563, PI18/01029, PI19/00553, and CIBERONC), the Generalitat de Catalunya (SGR 2017-449 and 2017-1282; and PERIS MedPerCan and URDCat), and the CERCA Program.
Author Contributions
L.P. and M.A.P. designed and performed the experiments. I.G.-F. and R.d.C. performed variant imputation in TCGA. L.P., R.E., E.B, and M.G.-V. performed bioinformatic analyses of genetic variants, PRSs, gene expression, and cell signatures. D.R.B. and A.C.A. performed association studies in BRCA1/2 mutation carriers. J. Beesley and G.C.-T. contributed to the association studies and performed candidate target gene analyses. M.G.-G., C.F., A.S., and A.I.E. contributed to the cohort study. D.O., A.R.-L., and C.V. contributed to the data normalization and multivariate regression designs. M.P.-C. and J.D. contributed to the pathway analyses. C.H., F.M., E.M., C.R., T.M., J. Brunet, R.H., and C.G. contributed data, evaluated the results, and critically revised the manuscript. C.L. contributed to the study design. M.A.P. wrote the paper.
Declaration of Interests
M.A.P. is recipient of an unrestricted research grant from Roche Pharma for the development of the ProCURE ICO research program. C.F. received support from Pfizer unrelated to this study.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101296.
Contributor Information
Antonis C. Antoniou, Email: aca20@medschl.cam.ac.uk.
Conxi Lázaro, Email: clazaro@iconcologia.net.
Miquel Angel Pujana, Email: mapujana@iconcologia.net.
Supplemental Information
References
- Astle W.J., Elding H., Jiang T., Allen D., Ruklisa D., Mann A.L., Mead D., Bouman H., Riveros-Mckay F., Kostadima M.A. The allelic landscape of human blood cell Trait variation and links to common complex disease. Cell. 2016;167:1415–1429.e19. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila Cobos F., Vandesompele J., Mestdagh P., De Preter K. Computational deconvolution of transcriptomics data from mixed cell populations. Bioinformatics. 2018;34:1969–1979. doi: 10.1093/bioinformatics/bty019. [DOI] [PubMed] [Google Scholar]
- Burnet F.M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. Weinstein J.N., Collisson E.A., Mills G.B., Shaw K.R.M., Ozenberger B.A., Ellrott K., Shmulevich I., Sander C., Stuart J.M. The cancer genome atlas pan-cancer analysis project. Nat. Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Juko-Pecirep I., Hammer J., Ivansson E., Enroth S., Gustavsson I., Feuk L., Magnusson P.K.E., McKay J.D., Wilander E., Gyllensten U. Genome-wide association study of susceptibility loci for cervical cancer. J. Natl. Cancer Inst. 2013;105:624–633. doi: 10.1093/jnci/djt051. [DOI] [PubMed] [Google Scholar]
- Cubuk C., Hidalgo M.R., Amadoz A., Pujana M.A., Mateo F., Herranz C., Carbonell-Caballero J., Dopazo J. Gene expression integration into pathway modules reveals a pan-cancer metabolic landscape. Cancer Res. 2018;78:6059–6072. doi: 10.1158/0008-5472.CAN-17-2705. [DOI] [PubMed] [Google Scholar]
- Dauphinee S.M., Clayton A., Hussainkhel A., Yang C., Park Y.-J., Fuller M.E., Blonder J., Veenstra T.D., Karsan A. SASH1 is a scaffold molecule in endothelial TLR4 signaling. J. Immunol. 2013;191:892–901. doi: 10.4049/jimmunol.1200583. [DOI] [PubMed] [Google Scholar]
- Ethier J.-L., Desautels D., Templeton A., Shah P.S., Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19:2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehringer G., Kraft P., Pharoah P.D., Eeles R.A., Chatterjee N., Schumacher F.R., Schildkraut J.M., Lindström S., Brennan P., Bickeböller H. Cross-cancer genome-wide analysis of lung, ovary, breast, prostate, and colorectal cancer reveals novel pleiotropic associations. Cancer Res. 2016;76:5103–5114. doi: 10.1158/0008-5472.CAN-15-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche L.G., Gruber S.B., Wu Z., Schmidt E.M., Zawistowski M., Moser S.E., Blanc V.M., Brummett C.M., Kheterpal S., Abecasis G.R., Mukherjee B. Association of polygenic risk scores for multiple cancers in a phenome-wide study: results from the Michigan Genomics Initiative. Am. J. Hum. Genet. 2018;102:1048–1061. doi: 10.1016/j.ajhg.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H., Hagerling C., Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Consortium The genotype-tissue expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra N., Tan Y.X., Joncker N.T., Choy A., Gallardo F., Xiong N., Knoblaugh S., Cado D., Greenberg N.M., Greenberg N.R., Raulet D.H. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry S.A., Farnsworth R.H., Solomon B., Achen M.G., Stacker S.A., Fox S.B. The role of the tumor vasculature in the host immune response: implications for therapeutic strategies targeting the tumor microenvironment. Front. Immunol. 2016;7:621. doi: 10.3389/fimmu.2016.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan T., Meng Q., Saleh M.A., Norlander A.E., Joehanes R., Zhu J., Chen B.H., Zhang B., Johnson A.D., Ying S. Integrative network analysis reveals molecular mechanisms of blood pressure regulation. Mol. Syst. Biol. 2015;11:799. doi: 10.15252/msb.20145399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung R.J., Ulrich C.M., Goode E.L., Brhane Y., Muir K., Chan A.T., Marchand L.L., Schildkraut J., Witte J.S., Eeles R. Cross cancer genomic investigation of inflammation pathway for five common cancers: lung, ovary, prostate, breast, and colorectal cancer. J. Natl. Cancer Inst. 2015;107:djv246. doi: 10.1093/jnci/djv246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabara H.H., Boyden S.E., Chou J., Ramesh N., Massaad M.J., Benson H., Bainter W., Fraulino D., Rahimov F., Sieff C. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat. Genet. 2016;48:74–78. doi: 10.1038/ng.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Sánchez A., Cast O., Miller M.L. Comprehensive benchmarking and integration of tumor microenvironment cell estimation methods. Cancer Res. 2019;79:6238–6246. doi: 10.1158/0008-5472.CAN-18-3560. [DOI] [PubMed] [Google Scholar]
- Jonsson S., Sveinbjornsson G., de Lapuente Portilla A.L., Swaminathan B., Plomp R., Dekkers G., Ajore R., Ali M., Bentlage A.E.H., Elmér E. Identification of sequence variants influencing immunoglobulin levels. Nat. Genet. 2017;49:1182–1191. doi: 10.1038/ng.3897. [DOI] [PubMed] [Google Scholar]
- Kasela S., Kisand K., Tserel L., Kaleviste E., Remm A., Fischer K., Esko T., Westra H.-J., Fairfax B.P., Makino S. Pathogenic implications for autoimmune mechanisms derived by comparative eQTL analysis of CD4+ versus CD8+ T cells. PLoS Genet. 2017;13:e1006643. doi: 10.1371/journal.pgen.1006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemann C., Wagner L., Stephan M., von Hörsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016;185:1–21. doi: 10.1111/cei.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten C., Fiore N., Trouw L.A., Csomor E., Xu W., Castellano G., Daha M.R., Gelderman K.A. Complement production and regulation by dendritic cells: molecular switches between tolerance and immunity. Mol. Immunol. 2008;45:4064–4072. doi: 10.1016/j.molimm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Kresovich J.K., O’Brien K.M., Xu Z., Weinberg C.R., Sandler D.P., Taylor J.A. Prediagnostic immune cell profiles and breast cancer. JAMA Netw. Open. 2020;3:e1919536. doi: 10.1001/jamanetworkopen.2019.19536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., He X., Schembri-King J., Jakes S., Hayashi J. Cloning and characterization of human Lnk, an adaptor protein with pleckstrin homology and Src homology 2 domains that can inhibit T cell activation. J. Immunol. 2000;164:5199–5206. doi: 10.4049/jimmunol.164.10.5199. [DOI] [PubMed] [Google Scholar]
- Lim Y.W., Chen-Harris H., Mayba O., Lianoglou S., Wuster A., Bhangale T., Khan Z., Mariathasan S., Daemen A., Reeder J. Germline genetic polymorphisms influence tumor gene expression and immune cell infiltration. Proc. Natl. Acad. Sci. U S A. 2018;115:E11701–E11710. doi: 10.1073/pnas.1804506115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Lichtenberg T., Hoadley K.A., Poisson L.M., Lazar A.J., Cherniack A.D., Kovatich A.J., Benz C.C., Levine D.A., Lee A.V. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173:400–416.e11. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis K.L., Rodabough R.J., Thomson C.A., Lopez A.M., McTiernan A., Women’s Health Initiative Research Group Prospective study of leukocyte count as a predictor of incident breast, colorectal, endometrial, and lung cancer and mortality in postmenopausal women. Arch. Intern. Med. 2007;167:1837–1844. doi: 10.1001/archinte.167.17.1837. [DOI] [PubMed] [Google Scholar]
- Maslah N., Cassinat B., Verger E., Kiladjian J.-J., Velazquez L. The role of LNK/SH2B3 genetic alterations in myeloproliferative neoplasms and other hematological disorders. Leukemia. 2017;31:1661–1670. doi: 10.1038/leu.2017.139. [DOI] [PubMed] [Google Scholar]
- Michailidou K., Lindström S., Dennis J., Beesley J., Hui S., Kar S., Lemaçon A., Soucy P., Glubb D., Rostamianfar A. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E., Tabarsi P., Mansouri D., Khosravi A., Garssen J., Velayati A., Adcock I.M. Cancers related to immunodeficiencies: update and perspectives. Front. Immunol. 2016;7:365. doi: 10.3389/fimmu.2016.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A.M., Steen C.B., Liu C.L., Gentles A.J., Chaudhuri A.A., Scherer F., Khodadoust M.S., Esfahani M.S., Luca B.A., Steiner D. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019;37:773–782. doi: 10.1038/s41587-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O., Zweigerdt R., Arnold H.H. Targeted disruption of the homeobox transcription factor Nkx2-3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development. 1999;126:2215–2225. doi: 10.1242/dev.126.10.2215. [DOI] [PubMed] [Google Scholar]
- Ribatti D. The concept of immune surveillance against tumors. The first theories. Oncotarget. 2017;8:7175–7180. doi: 10.18632/oncotarget.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalapour S., Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J. Clin. Invest. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A., Yurgelun M.B., Alexandrov L., Rao A., Bejar R., Polyak K., Giannakis M., Shilatifard A., Finn O.J., Dhodapkar M. Precancer atlas to drive precision prevention trials. Cancer Res. 2017;77:1510–1541. doi: 10.1158/0008-5472.CAN-16-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenina-Adognravi O. Invoking the power of thrombospondins: regulation of thrombospondins expression. Matrix Biol. 2014;37:69–82. doi: 10.1016/j.matbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A.M., Shih J., Ha G., Gao G.F., Zhang X., Berger A.C., Schumacher S.E., Wang C., Hu H., Liu J. Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell. 2018;33:676–689.e3. doi: 10.1016/j.ccell.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma A., Putluri N., Mishra P., Mathé E.A., Dorsey T.H., Yi M., Wallace T.A., Issaq H.J., Zhou M., Killian J.K. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J. Clin. Invest. 2014;124:398–412. doi: 10.1172/JCI71180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L. On immunosurveillance in human cancer. Yale J. Biol. Med. 1982;55:329–333. [PMC free article] [PubMed] [Google Scholar]
- Thorsson V., Gibbs D.L., Brown S.D., Wolf D., Bortone D.S., Ou Yang T.-H., Porta-Pardo E., Gao G.F., Plaisier C.L., Eddy J.A. The immune landscape of cancer. Immunity. 2018;48:812–830.e14. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkamani A., Wineinger N.E., Topol E.J. The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 2018;19:581–590. doi: 10.1038/s41576-018-0018-x. [DOI] [PubMed] [Google Scholar]
- Tsoi L.C., Stuart P.E., Tian C., Gudjonsson J.E., Das S., Zawistowski M., Ellinghaus E., Barker J.N., Chandran V., Dand N. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat. Commun. 2017;8:15382. doi: 10.1038/ncomms15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez L., Cheng A.M., Fleming H.E., Furlonger C., Vesely S., Bernstein A., Paige C.J., Pawson T. Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J. Exp. Med. 2002;195:1599–1611. doi: 10.1084/jem.20011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay D.S., Ryan E.P., Pawelec G., Talib W.H., Stagg J., Elkord E., Lichtor T., Decker W.K., Whelan R.L., Kumara H.M.C.S. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015;35 Suppl:S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Ward L.D., Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb G.J., Hirschfield G.M. Using GWAS to identify genetic predisposition in hepatic autoimmunity. J. Autoimmun. 2016;66:25–39. doi: 10.1016/j.jaut.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Zanotti K.J., Maul R.W., Castiblanco D.P., Yang W., Choi Y.J., Fox J.T., Myung K., Saribasak H., Gearhart P.J. ATAD5 deficiency decreases B cell division and Igh recombination. J. Immunol. 2015;194:35–42. doi: 10.4049/jimmunol.1401158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
TCGA data were obtained from the Genomic Data Commons Data Portal (https://portal.gdc.cancer.gov) and from the corresponding consortium publications. Individual genetic data were obtained following specific approval: dbGaP Data Access Committee project #11689. The R software algorithms developed by others and applied in this study are detailed in the Transparent Methods supplemental file. A complete pipeline was implemented and is available at https://github.com/pujana-lab/systematicQTL/.