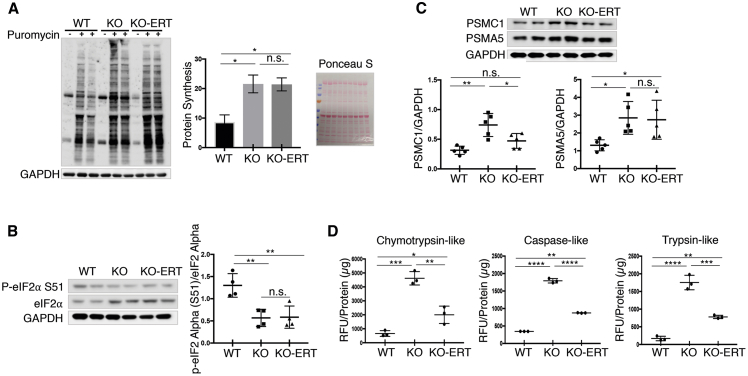
Figure 8.
AT-GAA Had a Modest Effect on Muscle Proteostasis in KO Mice
KO mice received 12 bi-weekly i.v. administrations of AT-GAA. Age and sex-matched WT and untreated KO mice were used for the comparisons. Muscle biopsies were collected 7–10 days after the last administration. (A) Surface sensing of translation (SUnSET) analysis was used to evaluate the rate of protein synthesis. The animals were injected intraperitoneally (i.p.) with puromycin (the aminoacyl-tRNA analog) 30 min prior to sacrifice. Western blot of muscle lysates with anti-puromycin antibody was then used to detect the incorporation of puromycin into nascent polypeptides. Total intensity of puromycin-labeled polypeptides was quantified. The increased protein translation is observed in both untreated and treated KO samples. Western blot with anti-GAPDH and Ponceau S staining were used as loading controls. (B) Western blot of muscle lysates from WT, untreated-, and treated KO muscle shows a decrease in the p-eIF2α S51/eIF2α ratio (consistent with the increase in protein synthesis) in both untreated and treated KO samples. (C) Western blot of total lysates from WT, untreated-, and treated KO muscle shows increased levels of proteasome 26S subunit, ATPase 1 (PSMC1), and alpha 5 (PSMA5) subunits in the KO; the level of PSMC1 returned to normal following ERT, whereas the level of PSMA5 did not (n = 5 for each condition. (D) The proteasome activity was measured in proteasome-enriched fractions isolated from WT, untreated-, and treated KO muscle extracts. The activity was significantly improved following ERT but still remained elevated compared to the WT controls. The results are shown in relative fluorescence units (RFU)/mg protein (n = 3 for each group). Note that the lysates for (B) and (C) were the same as those used in Figures 1, 2, 5,6, and 7 (n = 4–5 for each group). Data are mean ± SD. Statistical significance was determined by one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.